Silicon and Group 14 Elements
- Page ID
- 31758
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Predict some chemical reactions for a set of conditions.
- Describe some chemical and physical properties of \(\ce{C}\) and \(\ce{Si}\).
Group 14 Elements C, Si, Ge, Sn, Pb
Group 14 elements play more important roles in our lives and our civilization than elements of any other group. Thus, every educated person should know something about them.
- Carbon - The element of organic chemistry and life.
- Silicon - The element of information technology.
- Germanium - The element of transistor bases.
- Tin and lead - Elements known to alchemists.
Chemistry of Carbon
Carbon exists as diamond, graphite, fullerenes, and charcoal. Their structures are interesting; so are their properties. You probably know a lot about diamond and graphite, but the fullerenes were discovered after 1970, and this discovery has opened a door for a lot of interesting research. Read about them in books, magazines and journals. You might find yourself working with them some day.
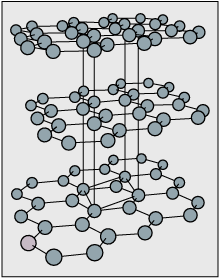
Among the fullerenes, one of the most common "molecules" has 60 \(\ce C\) atoms, and is represented by \(\ce{C60}\); a diagram is shown here. If you connect the 60 carbon atoms with bonds, the structure looks like a cage with 5- and 6- member rings. Synthesis, bonding, symmetry and stability of the cagelike fullerenes have already attracted a lot of attention, and their properties are even more fascinating.

Regarding carbon compounds, you already know something about \(\ce{CO2}\) and \(\ce{CO}\), including their roles in the environment. The hard carbides such as \(\ce{Fe3C}\), \(\ce{WC}\), and \(\ce{TiC}\) are more interesting to material scientists and engineers for their application in cutting tools. The calcium carbide \(\ce{CaC2}\) produced by reducing \(\ce{CaO}\) by carbon was a valuable commodity at one time due to its reaction with water to give acetylene gas:
\(\ce{CaC_2 + 2 H_2O \rightarrow Ca(OH)_2 + C_2H_{2\large{(g)}}}\)
Acetylene is an important industrial gas for the manufacture of polymers.
Silicon
Do you know that:
- \(\ce{Si}\) comprises 27.7 % of the Earth's crust?
- silicates, \(\ce{SiO2}\) based minerals, are everywhere?
- \(\ce{Si}\) is an essential element for bone growth?
- \(\ce{Si}\) crystals are the bases of computer chips?
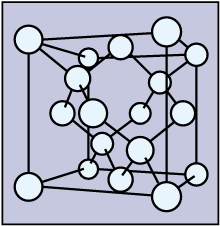
- the structure of \(\ce{Si}\) is the same as that of diamond, and this feature is important for computer chips?
- How we convert \(\ce{SiO2}\) into \(\ce{Si}\) element?
Silicates
By weight, silicon is the most abundant element in the Earth's crust. It usually exists in the form of an oxide, \(\ce{SiO2}\). This formula does not do justice to so many different materials we call silicates, but these substances are indeed \(\ce{SiO2}\). Some of the minerals contain impurities.
In pure form, \(\ce{SiO2}\) is quartz. Small particles of quartz are sand. They are hard. In the structure at the atomic level, every silicon atom is bonded to 4 oxygen atoms, and every oxygen is bonded to two \(\ce{Si}\) atoms. The four \(\ce{Si-O}\) bonds point towards the corners of a tetrahedron, as do the \(\ce{C-C}\) bonds in the diamond structure. When an impurity is present, the quartz may be colored. Due to various arrangements of the \(\ce{Si-O-Si}\) bonds, the same substance appears in many forms.
A basic unit of silicate structures is \(\ce{SiO4^4-}\). The gemstone zircon has a formula \(\ce{ZrSiO4}\), and olivine has a chemical formula of \(\ce{(MgFe)2SiO4}\). Two \(\ce{SiO4^4-}\) units combine to give the pyrosilicate unit \(\ce{Si2O7^6-}\), and it appears in akermanite, \(\ce{Ca2MgSi2O7}\). When the number of units increases, the tetrahedral units combine to form rings, chains, layers and 3-dimensional networks. Thus, the structure and classification of silicate is a major part of minerals. (This site has some interesting pictures.)
Silicon and Silane
Elemental silicon can be obtained from reduction of silicates. The reduction of sand, \(\ce{SiO2}\) by carbon at 3300 K in the reaction,
\(\ce{SiO_2 + 2 C \rightarrow Si_{\large{(l)}} + 2 CO} \;\;\; \textrm{at 3300 K}\)
gives liquid silicon. The silicon so obtained is usually not pure, and for the computer industry, the element must be purified. Crystal growth and silicon fabrication dominated the industry in the 1980s and 1990s, and perhaps into the next century, and the production of the element is only the beginning of the process.
If a more reactive element, \(\ce{Mg}\), is used in the reduction, \(\ce{Mg2Si}\) is formed,
\(\ce{SiO_2 + 4 Mg \rightarrow Mg_2Si + 2 MgO}\)
\(\ce{Mg2Si}\) is a compound, and it reacts with water to form silane.
Silane, \(\ce{SiH4}\), can be produced by reacting \(\ce{Mg2Si}\) with acids
\(\ce{Mg_2Si + 4 H_2O \rightarrow 2 Mg(OH)_2 + SiH_4}\)
and \(\ce{SiH4}\) is ignited when it contacts air, being much more reactive than methane,
\(\ce{SiH_4 + O_2 \rightarrow SiO_2 + H_2O}\)
In a basic solution, \(\ce{SiH4}\) reacts with water to give \(\ce{SiO(OH)3-}\),
\(\ce{SiH_4 + OH^- + 3H_2O \rightarrow SiO(OH)_3^- + 4 H_2}\)
Silicon Halides
Silicon tetrafluoride is formed when glass (\(\ce{SiO2}\)) is exposed to \(\ce{HF}\), and when \(\ce{Si}\) reacts with \(\ce{F2}\),
\(\ce{SiO2 + 4 HF_{\large{(aq)}} \rightarrow 2 H2O + SiF_{4\large{(g)}}}\)
\(\ce{Si + 2 F2 \rightarrow SiF_{4\large{(g)}}}\)
When chlorine passes through hot sand (\(\ce{SiO2}\)) and carbon, \(\ce{SiCl4}\) is produced,
\(\ce{SiO2 + C + 2 Cl_2 \rightarrow 2CO + SiCl_{4\large{(g)}}}\)
\(\ce{SiCl4}\) and \(\ce{SiF4}\) react with water to give silicic acid,
\(\ce{SiCl4 + 4H2O \rightarrow 4 HCl + Si(OH)_{4\large{(aq)}}}\),
\(\ce{SiF4 + 4H2O \rightarrow 4 HF + Si(OH)_{4\large{(aq)}}}\).
Silicone Polymers
Silicones are polymers with the general formula \(\mathrm{(R_2SiO_2)_{\large n}}\) or \(\mathrm{(RSiO_3)_{\large n}}\), (\(\ce{R}\) = \(\ce{CH3}\), \(\ce{C2H5}\), \(\ce{C6H5}\), etc). The chain is held together by \(\ce{Si-O-Si}\) bonds. A simple one is \(\mathrm{((CH_3)_2SiO_2)_{\large n}}\),
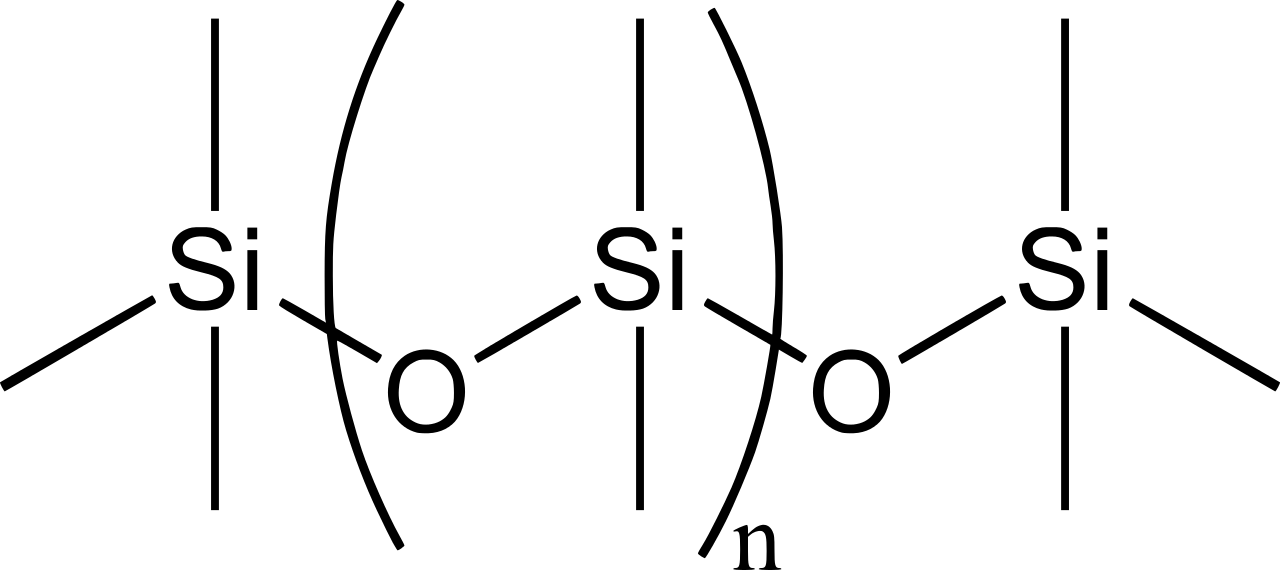
Figure 2: Chemical structure of the silicone polydimethylsiloxane (PDMS).
Of course, the 4 bonds around the \(\ce{Si}\) atoms point to the corners of a tetrahedron. These siloxane polymers are widely used as sealants, adhesives, additives, flame retardants, and lubricants. They have a wide application in industries. Depending on the organic group attached to silicon, the inorganic polymer silicones have been an important class of materials.
Questions
- Which one lists the group 14 elements in order of increasing atomic weight?
- \(\textrm{B Al Ga In Tl}\)
- \(\textrm{C Si Ge Sn Pb}\)
- \(\textrm{N P As Sb Bi}\)
- \(\textrm{O S Se Te Po}\)
- \(\textrm{F Cl Br I At}\)
- Which allotrope of carbon is the hardest: diamond, graphite, or fullerenes?
- What compound of carbon reacts with water to give acetylene gas?
- When you want to extract silicon element, what do you use to reduce the sand: \(\ce{SiO2}\), \(\ce{C}\) or \(\ce{Mg}\)?
- Which is stable towards air: methane or silane?
- Give the name of polymers whose chains are held together by \(\ce{Si-O-Si}\) bonds.
- In the crystal structure of \(\ce{Si}\), how many other \(\ce{Si}\) atoms are connected to a particular \(\ce{Si}\) atom?
Solutions
- Answer b
Hint...
Knowing the groups of elements enables us to correlate their chemical properties. Each list of choices is a group of elements on the period table.
a = 3A, b = 14, c = 5A, d = 6A, e = 7A. -
Answer diamond
Hint...
Diamond is the hardest thing in the world. Fullerenes are large molecules consisting of 40 to hundreds of carbon atoms, with \(\mathrm{C_{60}}\) being the most common. -
Answer . . .\(\ce{CaC2}\)
Hint...
The reaction to produce acetylene gas is\(\ce{CaC2 + H2O \rightarrow C2H2 + Ca(OH)2}\)
Acetylene is still an important industrial gas, as raw material for polymers.
-
Answer ...\(\ce{C}\)
Hint...
Carbon or coke is used for silicon metal, because \(\ce{Mg2Si}\) is formed if \(\ce{Mg}\) is used. -
Answer ... methane is stable
Hint...
Methane is the major component of natural gas, and it will not react with air until ignited, whereas silane ignites explosively as soon as it contacts air. -
Answer ...silicones
Hint...
Silicon polymers are an important class of materials invented not too long ago. - Answer ... 4
Hint...
Silicon and diamond have the same crystal structure. The edge of unit cells of \(\ce{Si}\) is larger than that of diamond.
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)

