1.18: Definition, Importance and History of Organometallics
- Page ID
- 204719
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
Organometallic chemistry, as defined by Dr. Brian W. Pfennig in his book Principles of Inorganic Chemistry, as “the chemistry of compounds that contain at least one metal–carbon bond (other than cyanide).” Though quite simplistic in definition, organometallic chemistry is a rather modern sub-discipline of chemistry when compared to its contemporary chemistry disciplines. Organometallic chemistry joins the of coordination complexes of inorganic chemistry with the synthetic methods of organic chemistry. Today’s interpretation of organometallic chemistry has now interwoven itself into many other disciplines; including bioorganometallic chemistry and catalytic chemistry. The former being a critical component in modern organic chemistry. So much so that without a organometallic catalyst, reaction such as the 2010 Nobel prize winning Heck reaction could not exist. While the latter is critical to life functions within organisms, such as the iron which coordinates the heme group in the blood cells that transfers oxygen to the tissues. Today, organometallic chemistry is used extensively in the modern world, from the construction of polymers, plastics, and petrol, to electronic circuitry and solar panel construction, to advances in medicine such as immunization inoculations and chemotherapy.[1]
History

French chemist Louis Claude Cadet de Gassicourt isolated the first organometallic compound, tetramethyldiarsine a.k.a. cacodyl, in 1757 by accident. He was experimenting with invisible inks by combining arsenic containing cobalt ore with potassium acetate. Arsenic itself is not a true metal, rather it is considered a metalloid, nonetheless it is still considered an organometallic compound.[2]
The first organometallic compound containing a transition metal was formed 67 years later by Danish organic chemist William Christopher Zeise by placing platinum tetrachloride in boiling ethanol. The resulting ion formed was trichloro-(ethene)-platinate (II) anion. When combined with a potassium counter ion, the Zeise salt is formed. The compound drew plenty of criticism in its day from Zeise colleagues over its actual structure. A problem that wasn’t solved until x-ray crystallography became available in the 20th century. Zeise’s salt kickstarted an interest in organometallic compounds even though the 19th century chemist did not exactly know why or how these compounds form. Compounds such as diethyl zinc and the extremely toxic nickel tertracabonyl were formed in the later half of the 19th century. With the former, synthesized by British chemist Ludwig Mond, initiating an entire new class of compounds called metal carbonyl’s.[3]
At the turn of the 20th century French chemist Victor Grignard discovered a new method of coupling carbon to the carbonyl group of a ketone/aldehyde by nucleophilic addition using an alkyl/aryl halide coupled to magnesium metal. Grignard’s groundbreaking organometallic reagent, which now bears his name, swept through the chemistry labs of the early 20th century and was awarded the 1912 Nobel Prize of Chemistry in addition with Paul Sabatier. Over one hundred years later it is still used extensively as a coupling reagent to a variety of carbonyl derivatives.[4]

The following year, in 1913, the Nobel in chemistry went to Swiss inorganic chemist Alfred Werner for his work involving the coordination chemistry of ligands to metals. In particular the structure of hexaminecolbalt (III) chloride. Werner’s work in coordination chemistry proved to be vital in the understanding of organometallic coordination and chemical reactions of compounds and contributed greatly to opening up the organometallic discipline.Though many new organometallic compounds were being created and used, organometallic chemistry was still not recognized as its own independent sub-discipline of chemistry until half way into the 20th century and the discovery of ferrocene in 1951.[5]
Ferrocene was created in 1951 by American chemists Peter Pauson and Tom Kealy by reacting cyclopentadiene magnesium bromide and ferric chloride together, resulting in an orange powder now known as ferrocene. Unfortunately for the two chemists they did not deduce the actual structure of their organometallic salt and erroneously proposed that the iron acted as a bridge in-between the first carbon of two cyclopentadiene molecules. Later on, English chemist Sir Geoffrey Wilkerson, in collaboration with American chemist Robert Woodward, Figured out that the iron in ferrocene was actually being sandwiched in between two cyclopentadiene molecules. In ferrocene, each cyclopentadiene achieves aromaticity and all 12 electrons covalently bond with the iron atoms available sigma and pi orbitals creating a very stable 18 electron containing molecule. Independently, German chemist Ernst Fischer also came to the same conclusions of the sandwich model for ferrocene. Fisher realized that this sandwich compound was not a result of the metal used, but rather how the coordination of the interaction of the cyclopentadiene ligand and metal occurred. Fisher then expanded the metallocene compounds to include other metals. Together, Wilkerson and Fisher shared the 1973 Nobel Prize of Chemistry for their respective work with metallocenes.[6]
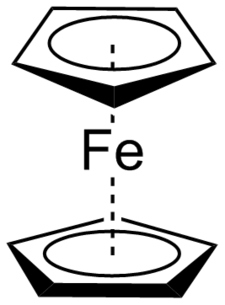
The discovery and understanding of metallocenes officially brought forth organometallic chemistry into to its own sub-discipline of chemistry. In doing so, opened up an explosion of new ideas of how to use organometallic compounds. Though one use stood out from the rest, that being to use organometallic compounds as catalyst’s in reactions. One early catalytic organometallic compound, dicyclopentadiene zircon (IV) dichloride was created jointly by chemist Karl Ziegler and Giulio Natta to polymerize terminal olefins. This actual led to two entire classes of organometallic catalyst’s, now known as Ziegler-Natta catalysts and earned each a Nobel Prize of Chemistry in 1963. Armed with new organometallic catalysts, chemists of the late 20th century designed new ways to couple carbons together. These include the famed Heck reaction, the Sharpless epoxidation, and the Grubbs olefin metathesis. Each of which earned a Nobel Prize of chemistry in 2010, 2001, and 2005 respectively.[7]
References
- Pfennig, B. W. (2015). Principles of Inorganic Chemistry (pp. 627-628). Hoboken, NJ: John Wiley & Sons, Inc.
- Seyferth, D. (2001). Cadet's Fuming Arsenical Liquid and the Cacodyl Compounds of Bunsen. Organometallics, 1488-1498. doi:10.1021/om0101947
- Hunt, L.B. (1984). The First Organometallic Compounds. Platinum Metals Rev, 28(2), 76-83.
- Hodson, D. (1987). "Victor Grignard (1871-1935)". Chemistry in Britain. 23: 141–2.
- Hunt, L.B. (1984). The First Organometallic Compounds. Platinum Metals Rev, 28(2), 76-83.
- Werner, H (2012). "At Least 60 Years of Ferrocene: The Discovery and Rediscovery of the Sandwich Complexes". Angew. Chem. Int. Ed. 51: 6052–6058. doi:10.1002/anie.201201598.
- Pfennig, B. W. (2015). Principles of Inorganic Chemistry (pp. 627-628). Hoboken, NJ: John Wiley & Sons, Inc.


