Olefin Metathesis
- Page ID
- 69099
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)As you may have learnt in your general chemistry course, metathesis is the exchange of atoms or functional groups in the substrates and the rearrangement of their matching partners to form new compounds. Simple examples were well-studied in traditional inorganic chemistry, but their analogs in organic reactions were vague and hard to proceed without suitable catalysts.
eg:
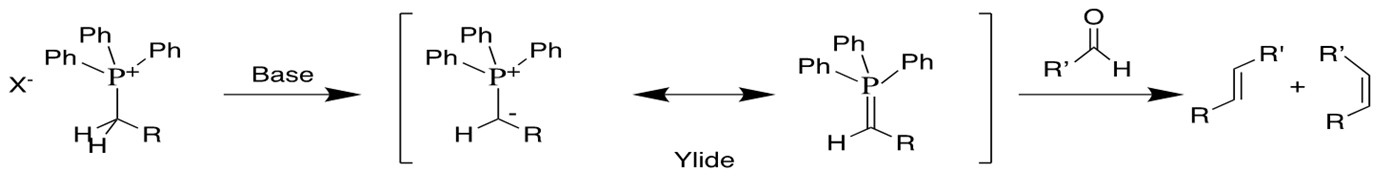
Olefin Metathesis:
The Nobel Prize in Chemistry of 2015 was shared by Yves Chauvin, Robert H.Grubbs and Richard R.Schrock for their contributions to the field of Olefin Metathesis.
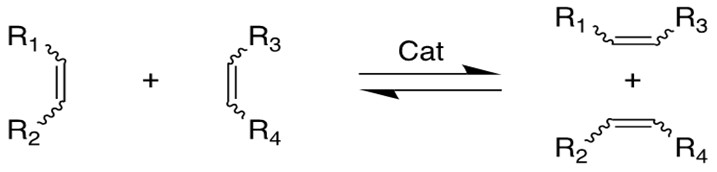
Olefin Metathesis[1] involves two olefin substrates which form a four-membered ring intermediate (first proposed by Chauvin) and then rearrange the substituents to form two new carbon-carbon double bonds. Late transition metal alkylidene compounds (eg. Grubbs Ru catalyst) greatly broaden the methodology to form carbon-carbon double bonds with high functional group tolerance. Schlock designed and synthesized another group of Mo,W and Re catalysts which exhibit higher efficiency for this reaction[2][3].

From left to right: Yves Chauvin, Robert H.Grubbs and Richard R.Schrock
Catalysts used for this reaction [4] [5a-g]:
- The earliest heterogenous catalytic system is composed of high-valent transition metal halide, oxide and oxo-halide. Alkyl zinc or alkyl aluminum is used as the co-catalyst. Also, aluminum oxide or silica is added as support.
Characteristics:
- Good Reactivity
- Extremely poor functional group tolerance
(Why? Because it is a lewis acid, it will be poisoned)
- Complicated and ambiguous catalytic mechanisms
2. Titanocene-based catalyst as Tebbe’s reagent is transformed into “Cp2Ti=CH2” in the presence of base. And it acts as an active metathesis catalyst.
Characteristics:
- Mild Reactivity
- Resemble stoichiometric Wittig-Like reactions
- Mechanism resembles Olefin Metathesis except the irreversibility of the last step
(What’s the limitation of this method? It can only perform methylenation, not alkylidenation.)
3. Schrock pioneered the preparation of a series of W, Mo and Re catalysts for olefin metathesis reactions. And the Mo complex with arylimido ligand stands out with impressive activity and high turnover frequency[6a,b].
Characteristics:
- Lower functional group tolerance
- Air and water sensitive
- Multi-substituted and hindered substrates accessible
(Why high reactivity? Because the coordinative and electronic unsaturation along with the bulky ligands make them good electrophilic agents and reduce the bimolecular decomposition)

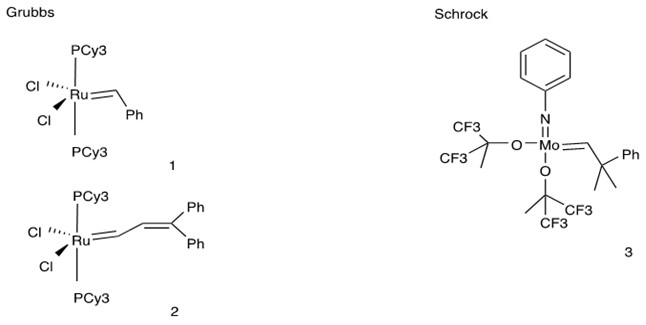
4. Grubbs later rationalized the design and synthesized another group of Ru-based catalysts with unique properties. His 2nd generation of catalyst employed the nitrogen heterocyclic carbene which facilitated the dissociation of trans-ligands. Hoveyda collaborated with Grubbs to make a system with chelating ligands which was more applicable to sterically demanding and electron-poor substrates [7][8].
Characteristics:
- High functional group tolerance
- Stable in water conditions
- Lower reactivity than Schrock’s catalyst

Comparison Between Grubbs and Schrock Catalysts [9][10]:
|
Catalyst |
Grubbs |
Schrock |
|---|---|---|
|
Reactivity |
Low |
High |
|
Substrate |
Less bulky and strained alkenes |
Sterically-demanding and electron-deficient alkenes |
|
Rxn Condition |
Stable in bench conditions |
Sensitive to air and water |
|
Functional Group Tolerance |
High |
Poor |
General Mechanism:
Chauvin incubated the earliest imaginary mechanism involving a [2+2] cycloaddition reaction between the transition metal alkylidene complex and the olefin substrate to form a four membered ring metallacyclobutane intermediate. Then the metallacycle breaks up in an opposite fashion to yield both the new alkylidene and the olefin product.
According to the traditional organic chemistry using MO theory, such a ring addition reaction is symmetrically forbidden and requires photochemical condition to proceed. However, the introduction of metal alkylidene fragment with d-orbitals breaks the symmetry and facilitates the reaction.
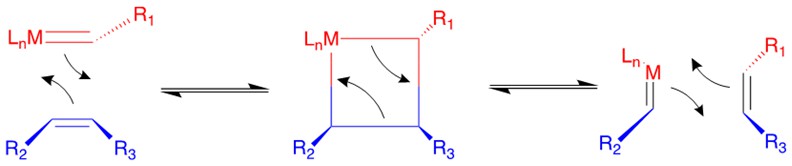
Categories of Olefin Metathesis:
1. Cross Metathesis
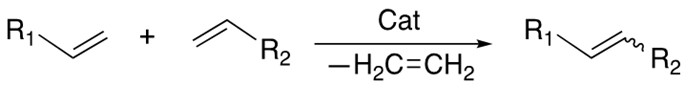
The transalkylidenation of two terminal alkenes with release of ethene is catalyzed by the Grubbs catalyst. Both homocoupling and heterocoupling can occur and the E/Z selectivity is hard to control.
Mechanism [4]:
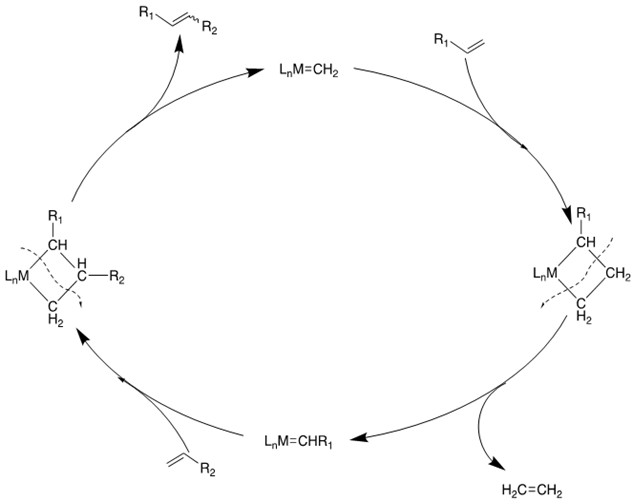
2. Ring-Closing Metathesis
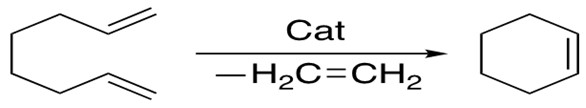
Ring-closing metathesis allows formation of cyclic alkenes ranging from 5 to 30 members, in which the E/Z selectivity is related to the ring strain. The 2nd generation Grubbs catalyst is more versatile for this reaction.
Mechanism:
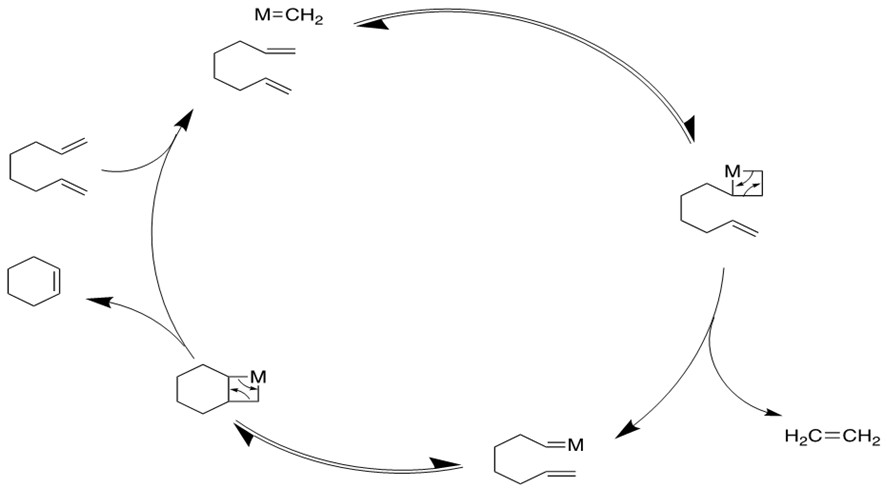
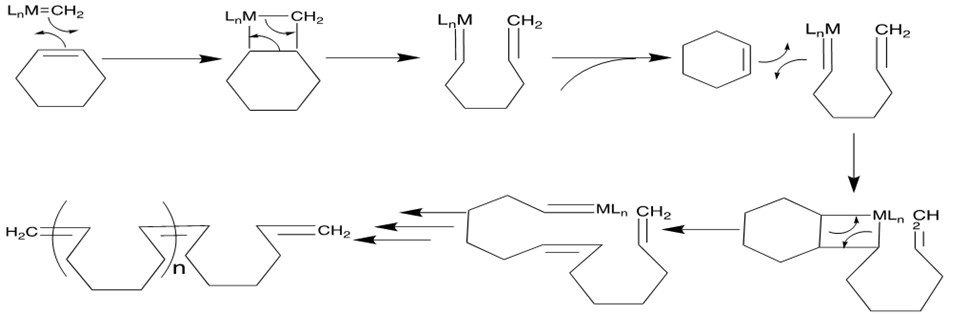
3. Ring-Opening Polymerization Metathesis (ROMP) [11a-c]
Ru based catalysts can open the strained ring with a second alkene via the cross-metathesis mechanism to form products containing terminal vinyl groups. Further metathesis can occur to form long polymer chains.
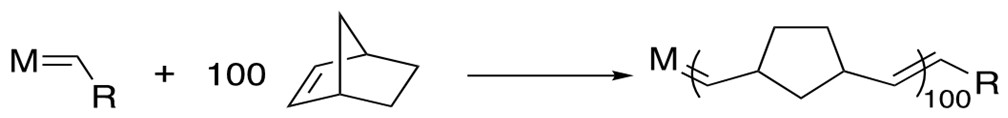
Mechanism[12a]:
4. Enyne Metathesis[12b-c]
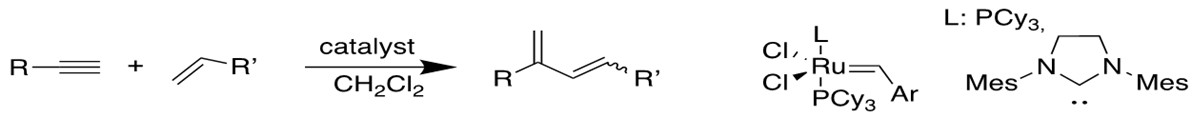
Alkyne and alkene can have similar reaction to produce 1,3-diene, and this intermolecular process is called cross-enyne metathesis, whereas the intramolecular reactions are referred to as ring-closing anyone metathesis(RCEYM).
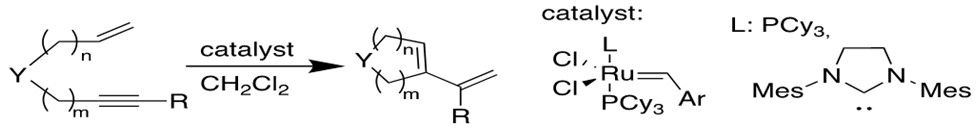
5. Acyclic Diene Metathesis Polymerization(ADMET)[12d]
Alpha-omega dienes can be used to produce polymers through ADMET method, and the reverse of this reaction has been studied as a potential means to recycle rubber tires.
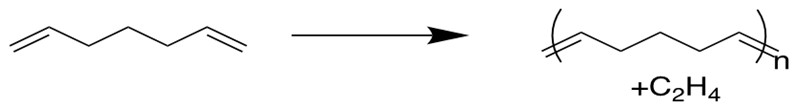
(What’s the driving force for these reactions? To form volatile alkenes or to release ring strain)
Detailed mechanistic studies of Grubbs group catalysts in Cross Metathesis[13]
Classification of Olefins:
|
Type I:Fast homodimerization Olefins homodimerize rapidly and both the homodimers and monomers can enter the catalytic CM cycle. eg. Terminal Olefins Primary allylic alcohols Esters |
Decreasing in Olefin Reactivity. Increasing Steric Congestion. |
|
Type II:Slow homodimerization Slow olefin homodimerization and the homodimers can be only partly consumed in latter CM catalytic reactions. eg. Styrene Secondary allylic alcohols |
Increasing Electron Deficiency. |
|
Type III:No homodimerization Substrates can’t homodimerize but can facilely undergo CM with other type I or type II olefins. eg. Vinyl siloxanes |
|
|
Type IV:Spectators Don’t participate CM catalysis but also don't block the catalyst’s activity toward other olefins. eg. Quaternary allylic carbon-containing olefins |
Hint: Some reactants are called bridge type olefins and the reactivity depends on the substituent pattern and catalyst choices. In other words, these substrates can be classified into different groups depending on the reaction conditions.
Reactivity Matrix for Cross Metathesis[13][14][15]:
|
ST: Statistical, selectivity achieved by excessive amount of one substrate(10 equiv). S: Selective, selectivity achieved by intrinsic thermodynamics. SI: Slow reaction. NR: No reaction. NS: Non-selective |
|
Reactivity Matrix for Cross Metathesis |
||||
|---|---|---|---|---|
|
I |
ST |
|||
|
II |
S |
NS |
||
|
III |
S |
SI |
NS |
|
|
IV |
NR |
NR |
NR |
NR |
|
I |
II |
III |
IV |
|
Pros and Cons of Cross Metathesis Reactions:
|
Pros |
Cons |
|
Excellent functional tolerance Commercially available olefin substrates Low catalyst loading Mode of selectivity makes reactivity predictable |
Model of E:Z unclear Intrinsic cross product selectivity remains problematic For complex molecules, unpredictable |
References
[1]: Skupinska, J. J.Chem.Educ. 1988, 65, 605.
[2]: Chem. & Eng. News. 2002, Dec 23, 29-33.
[3]: Calderon, N. et al; J. Polym. Sic., Part A1. 1967, 5, 2209-2217.
[4]: Hérisson, P. J.; Chauvin, Y. Die Makromolekulare Chemie. 1971, 141, 161-176.
[5]: a) McGinnis, J.; Katz, T. J.; Hurwitz, S. J. Am. Chem. Soc. 1976, 98, 605-606.; b) Schrock, R. R.; Murdzek, J. S.; Bazan G. C.; Robbins, J.; DiMare, M.; O'Regan, M. J. Am. Chem. Soc. 1990, 112, 3875-3886.; c) Nguyen, S. T.; Johnson, L. K.; Grubbs, R. H.; Ziller, J. W. J. Am. Chem. Soc. 1992, 114. 3974-3975.; d) Scholl, M.; Ding, S.; Lee, C. W.; Grubbs, R. H. Org. Lett. 1999, 6, 953-956.; e) Van Veldhuizen, J. J.; Garber, S. B.; Kingsbury, J. S.; Hoveyda, A. H. J. Am. Chem. Soc. 2002, 124, 4954-4955.; f) Stewart, I. C.; Douglas, C. J.; Grubbs, R. H. Org. Lett. 2008, 10, 441-444.; g) Tebbe, F. N.; Parshall, G.W.; Reddy, G.S. J. Am. Chem. Soc. 1978, 100, 3611.
[6]: a) Ibrahem, Ismail, et al. J.Am.Chem.Soc. 2009,131,3844; b) Fürstner, A. Nature. 2011,471,461
[7]: Nicolaou, K. C., et al. J.Am.Chem.Soc.1996,118,100-110
[8]: Vougioukalakis, G. C., & Grubbs, R. H. Chem. Rev. 2010,110,1746-1787
[9]: Nobel Chemistry Prize Lectures. Grubbs’ and Schrock’s
[10]: K. Grela, S. Harutyunyan, A. Michrowska, Angew. Chem. Int. Ed., 2002, 114, 4038.
[11]: a) Blechert et al. Angew. Chem. Int. Ed. 1997, 36, 257.; b) Snapper et al. J. Am. Chem. Soc. 1995, 117, 9610.; c) Tallarico, J. A.; Randall, M. L. Tetrahedron. 1997, 48, 16511.
[12]: a) Finkel'shstein et al. J. Mol. Cat. 1992, 76, 133. Forbes et al.; b) Katz et al. J. Am. Chem. Soc. 1985, 107, 737.; c) Mori et al. Synlett. 1994, 1020.; d) Forbes, Malcolm DE, et al. J. Am. Chem. Soc. 1992, 114, 10978.
[13]: Chatterjee, Arnab K., et al. Journal of the American Chemical Society. 2003, 125.37, 11360-11370.
[14]: “Olefin Cross Metathesis” Group Meeting by Dr.Pulin Wang from Prof.Burke Group
[15]: “Olefin Metathesis in Organic Synthesis” Group Meeting by Dr.Wendy Jen from Prof.MacMillan Group
Contributors
- Zhe Xu and Jianing Xu

