24.16.3: Organozinc Chemistry
- Page ID
- 85467
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In this lecture you will learn the following
- Organometallic compounds of zinc and cadmium.
- Structural features of organozinc compounds.
Organometallic compounds of zinc and cadmium
Dialkyl compounds of Zn, Cd and Hg do not associate through alkyl bridges. Dialkylzinc compounds are only weak Lewis acids, organocadmium compounds are even weaker, and organomercury compounds do not act as Lewis acids except under special circumstances.
The Group 12 metals form linear molecular compounds, such as ZnMe2, CdMe2 and HgMe2, that are not associated in solid, liquid or gaseous state or in hydrocarbon solution.
They form 2c, 2e bonds. Unlike Be and Mg analogs, they do not complete their valence shells by association through alkyl bridges. The bonding in these molecules are similar to d10 metals such as CuI, AgI and AuI with linear geometry ([N≡C-M-C≡N]-, M = Ag or Au). This tendency is sometimes rationalized by invoking pd hybridization in the M+ ion, which leads to orbitals that favor linear attachment of ligands (similar to spd hybridization).

The preference for the linear coordination may be due to the similarity in energy of the outer ns, np and (n-1)d orbitals, which permits the formation of collinear spd hybrids.
The hybridization of s, pz and dz2 with the choice of phases shown here produces a pair of collinear orbitals that can be used to form strong σ-bonds.
Organozinc and organocadmium compounds
Convenient route is metathesis with alkylaluminium or alkyllithium compounds.
With alkyllithium compounds it is the electronegativity which is decisive, whereas between Al and Zn it is hardness considerations correctly predict the formation of softer ZnCH3 and harder AlCl pairs.
![]()
Alkylzinc compounds are pyrophoric and readily hydrolyzed, whereas alkylcadmium compounds react more slowly with air. Due to mild Lewis acidity, dialkylzinc and dialkylcadimum compounds form stable complexes with amines, especially with chelating amines.
The Zn—C has greater carbanionic character than the Cd—C bond.
For example, addition of alkylzinc compounds across the carbonyl group of a ketone:
![]()
This reaction do not proceed with the less polar alkylcadmium or alkylmercury compounds, but organolithium, organomagnesium and organoaluminium compounds can promote this reaction readily since all of which contain metals with lower electronegativity than zinc.
Interestingly, the cyclodipentadienyl compounds are structurally unusual. CpZnMe is monomeric in the gas phase with a pentahapto Cp group.
In the solid state it is associated in a zig-zag chain, each Cp group being pentahapto with respect to two Zn atoms. 
Problems:
- Do you think that the following reaction proceeds? If so, why and how?

Solution:
Al2Me6 being an electron deficient molecule readily exchanges two methyl groups with zinc for two chloride ions. Since chloride ions have sufficient electron in their valence shell act as four electron donor through bridging coordination mode. Al2Cl2Me4 is no longer an electron deficient molecule.
Diethyzinc is the cheapest available organozinc compound and accounts for 60% of all references to diorganozinc reagents. The remainder coming from dimethyl (21%), dibutyl (4%), diisopropyl (4%), diphenyl (5%) and others (6%).
The different methods for preparing diorganozinc compounds can be grouped into four general approaches:
(a) the oxidative addition between zinc metal and an alkyl halide followed by Schlenk equilibration,
\[\ce{ 2 RX + 2 Zn → R2Zn + ZnX2}\]
(b) the transmetallation of a zinc halide with an organometallic reagent,
\[\ce{ 2 RM + 2 ZnX2 → R2Zn + 2 MX}\]
(c) the transmetallation of a diorganozinc starting material with an organometallic reagent, and
\[\ce{ 2 RM + R'2Zn → R2Zn + 2 R'M}\]
(d) the zinc-halogen exchange between an alkyl halide and a diorganozinc
\[\ce{2 RX + R'2Zn → R2Zn + 2 R'X}\]
Frankland prepared Et2Zn from EtI and Zn metal via EtZnI by distilling the more volatile Et2Zn which shifted the Schlenk equilibrium towards further product. No reaction occurred until 150 °C, but at 200 °C the Zn/EtI reaction proceeded with "tolerable rapidity", giving white crystals and leaving a colourless, mobile liquid. The sealed tube containing this reaction mixture remained sealed for some months because the only eudiometer required for the combustion of the product gases for their analysis was destroyed. In October 1848, Frankland returned to the University of Marburg to the laboratory of Robert Bunsen in order to obtain his Ph.D. under Bunsen's guidance. He took along with him the sealed tube from his EtI/Zn experiment at Queenwood College, Hampshire, England. He prepared the methyl derivative at about the same time and the apparatus shown in the paper reporting their preparation shows just how imaginative Frankland had become to cope with the air and moisture sensitivity of the products.
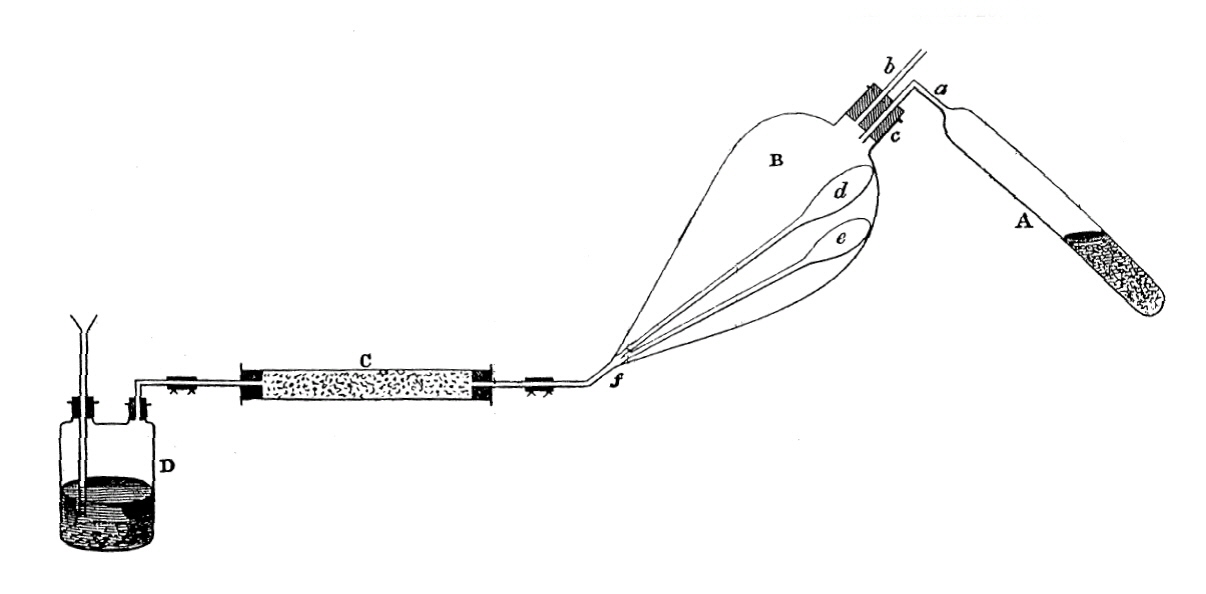
Apparatus for the preparation of diorganozinc compounds. Section A was where MeI was heated, B is the receiver containing the sample tubes d and e, C was a CaCl2 drying tube to prevent moisture and D was for generation of gaseous H2 to flush out and rid the system of air.
Frankland described these procedures in such detail to illustrate the painstaking, time-consuming care required in those days when working with extremely air and moisture-sensitive liquids. In 1877 Frankland noted in a 1000 page English translation of his papers that when
"I cut off the upper part of the tube in order to try the action of water upon the solid residue. On pouring a few drops of water upon the residue, a green-blue flame, several feet long, shot out of the tube, causing great excitement among those present. Professor Bunsen, who had suffered from arsenical poisoning during his research on cacodyl, suggested that the spontaneously inflammable body, which diffused an abominable odour through the laboratory, was that terrible compound, which might have been formed by arsenic present as an impurity in the zinc used in the reaction, and that I might be already irrecoverably poisoned. These forebodings were, however, quelled in a few minutes by an examination of the black stain [which was zinc] left upon porcelain by the flame; nevertheless, I did afterward experience some symptoms of zinc poisoning."
Dimethyl- and diethylzinc were the first available sources of nucleophilic alkyl groups, useful for the alkylation of inorganic and organometallic halides and of organic C=O and C=N-containing electrophiles. Early examples of the alkylation of metal-halogen functions were described by Frankland and others, and the dialkylzincs have been useful in this application ever since. Since they are less reactive than the comparable Grignard and organolithium reagents, they are particularly useful in the partial alkylation of element polyhalides, e.g., \(\ce{PCl3 → RPCl2}\).
The first use of a dialkylzinc in organic synthesis appears to have been reported by Freund of the University of Lemberg in 1861. In his search for a synthesis of ketones, he added acetyl chloride to diethylzinc. He had a difficult time obtaining a pure product but finally identified it as CH3C(O)Et.
\[\ce{2 CH3C(O)Cl + Et2Zn → 2 CH3C(O)Et + ZnCl2}\]
In 1867 Butlerow, in Russia, prepared tertbutyl alcohol by reaction of 2 equiv of dimethylzinc with acetyl chloride on a large scale, using 250 g of dimethylzinc. In this paper, Butlerow disputes Frankland's report of the toxic effects of dimethylzinc, saying that he and co-workers had worked with this compound for 5 years without taking any special precautions and never had a problem. He noted that smoking in the presence of dimethylzinc vapors (or, more correctly, vapors of the oxidation and hydrolysis products of dimethylzinc) changes the taste of the tobacco; it becomes unpleasantly sweetish!!
Following the discovery of the usefulness of Grignards in organic synthesis there was a decrease in the studies on Zn compounds until about 1984 when it was noted that enantioselective reactions could be performed using diorganoZn compounds.
Addition reaction of organozinc compounds to carbonyl compounds
The Barbier reaction (1899) is an organic reaction between an alkyl halide and a carbonyl group as an electrophilic substrate in the presence of magnesium, aluminium, zinc, indium, tin or its salts. The reaction product is a primary, secondary or tertiary alcohol. The reaction is similar to the Grignard reaction but the crucial difference is that the Barbier reaction is a one-pot synthesis whereas a Grignard reagent is prepared separately before addition of the carbonyl compound. Barbier reactions are nucleophilic addition reactions that usually take place with relatively inexpensive and water insensitive metals or metal compounds in contrast to Grignard reagents or organolithium reagents. For this reason it is possible in many cases to run the reaction in water which makes the procedure part of green chemistry. On the downside organozincs are much less nucleophilic than Grignards. The Barbier reaction is named after Victor Grignard's teacher Philippe Barbier.
An example of the Barbier reaction is the reaction of allyl bromide with benzaldehyde and zinc powder in water:
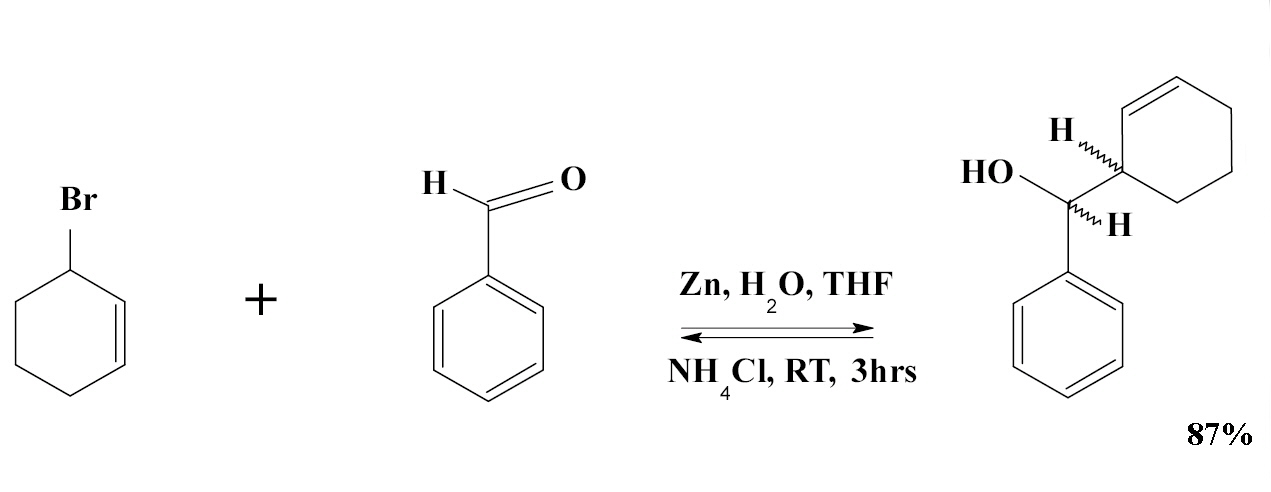
Barbier Reaction: The observed diastereoselectivity for this reaction is erythro : threo = 83 : 17
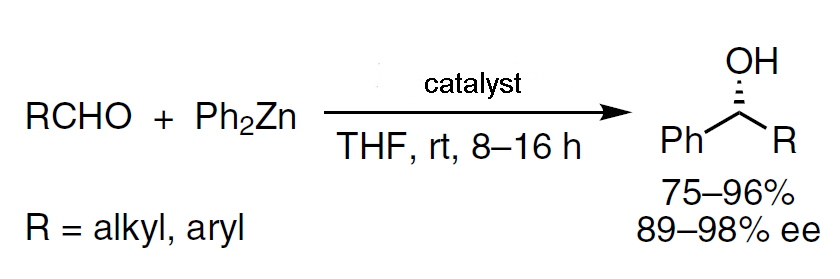
The catalytic enantioselective alkylation of aldehydes to afford enantioenriched secondary alcohols has been extensively studied since the 1980s. Diorganozinc reagents were the most widely used nucleophiles in this reaction, due to their low propensity to add to carbonyl derivatives without the presence of a suitable catalyst. A wide range of catalysts were investigated to optimise the conditions and conversion and to obtain the secondary alcohols in high enantiomeric excess.