16.4A: Dioxygen
- Page ID
- 34265
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Oxygen is a member of the chalcogen group (Group 16) of the periodic table and is a highly reactive nonmetallic element and oxidizing agent that readily forms compounds (notably oxides) with most elements. By mass, oxygen is the third-most abundant element in the universe, after hydrogen and helium. At standard temperature and pressure, STP, two atoms of the element bind to form dioxygen, O2, a diatomic gas that is colourless, odourless, and tasteless.
Discovery
Oxygen was discovered independently by Carl Wilhelm Scheele, in Uppsala, Sweden in 1773 or earlier, and Joseph Priestley in Wiltshire, UK in 1774, but Priestley is generally given the credit since his work was published first. The name oxygen was coined in 1777 by Antoine Lavoisier, whose experiments with oxygen helped to discredit the then-popular phlogiston theory of combustion and corrosion. Its name derives from the Greek roots οξυζ oxys, "acid", literally "sharp", referring to the sour taste of acids and -γενης genes meaning "creator", because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition.
Allotropes of oxygen
There are 2 main allotropes of oxygen although a third has recently been shown to form under high pressures.
|
|
|
|
|
|
| O2 - dioxygen | O3 - ozone | O8 - red oxygen |
Dioxygen - O2
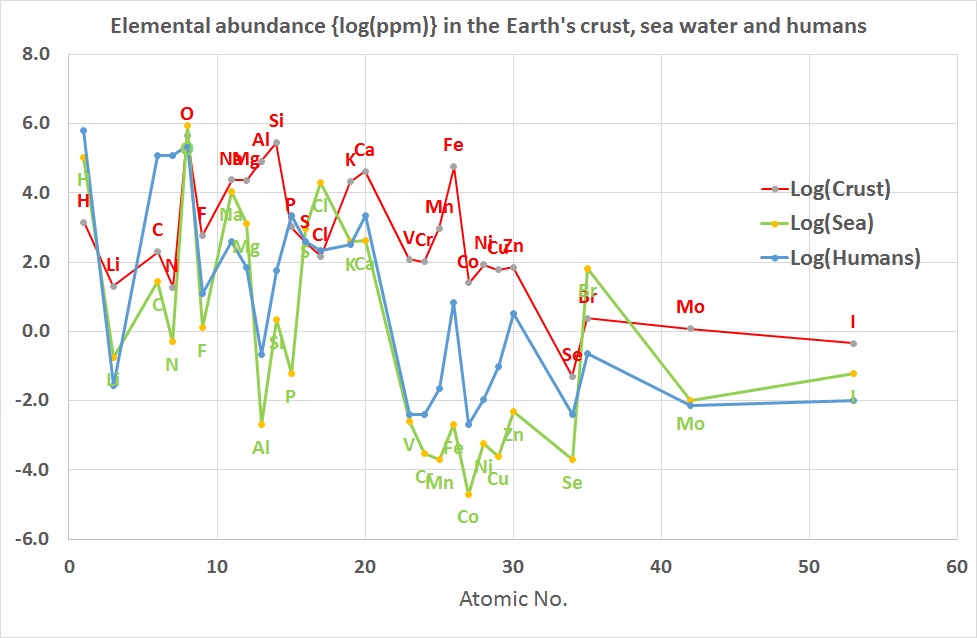
The common allotrope of elemental oxygen is often just called oxygen, O2, but to help distinguish it from the element may be called dioxygen or molecular oxygen. Elemental oxygen is most commonly encountered in this form, as about 21% (by volume) of the Earth's atmosphere is O2, the remainder largely being dinitrogen, N2. At STP, dioxygen is a colourless, odourless gas, in which the two oxygen atoms are chemically bonded to each other giving rise to two unpaired electrons occupying two degenerate molecular orbitals. The electron configuration of O2 molecules in this form, a diradical, indicates that they should be paramagnetic. This is a classic example of where a simple Lewis structure fails to account for the properties and where an MO approach correctly provides the explanation.
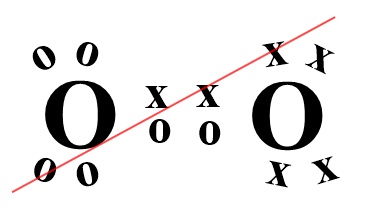
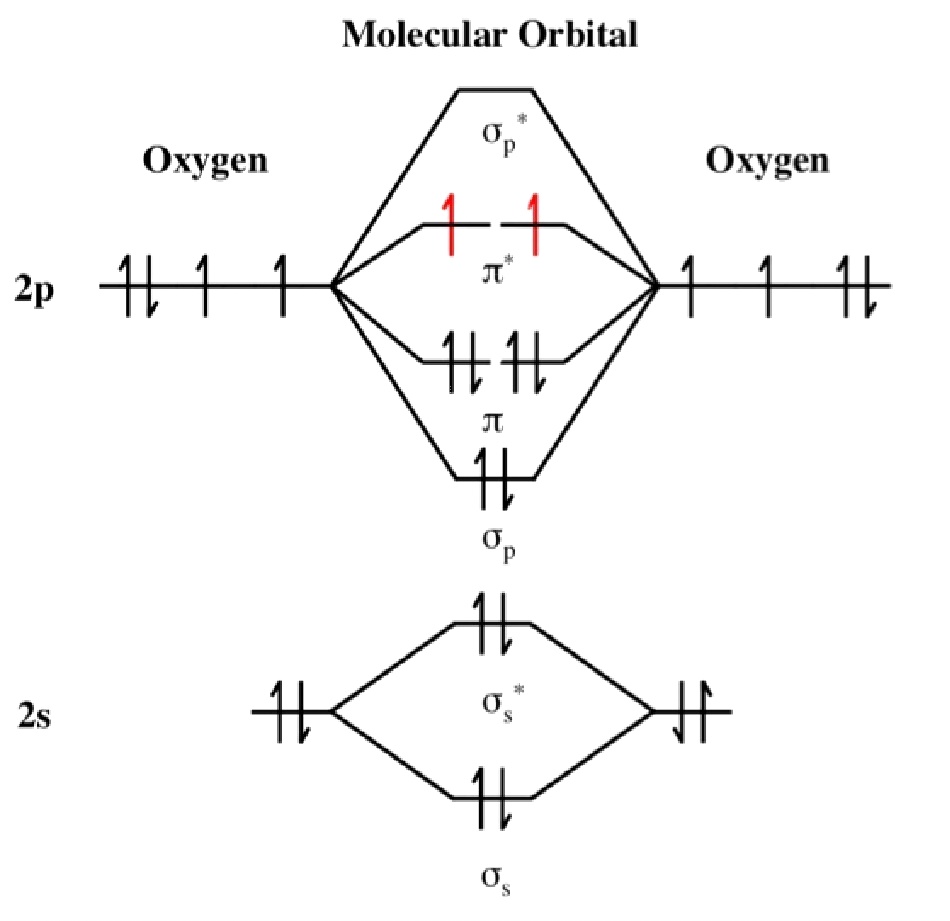 The electron configuration (ignoring 1s orbitals) is:
The electron configuration (ignoring 1s orbitals) is:(σ2s)2 (σ2s*)2 (σ2p)2 (π2p)4 (π2p*)2 and from this the Bond Order is found to be ½(2 -2 + 2 + 4 -2 ) = 2 that is, a double bond as shown in the Lewis structure as well. The difference though is that the Lewis structure does not predict the molecule to be paramagnetic. The bond length is 121 pm and the bond energy is 498 kJmol-1.
A video clip showing liquid dioxygen being poured between the faces of a magnet and attracted into the magnet field has been prepared as a Harvard Natural Sciences Lecture Demonstration.
With 2 electrons to be placed in 2 degenerate orbitals, a number of variations are possible and the arrangement above where the 2 electrons are parallel is considered to be the most stable. Note that the spin multiplicity is given by the formula, 2S+1 and so for S=1 from s=½ + ½ then 2S+1 = 3 i.e. a spin triplet.
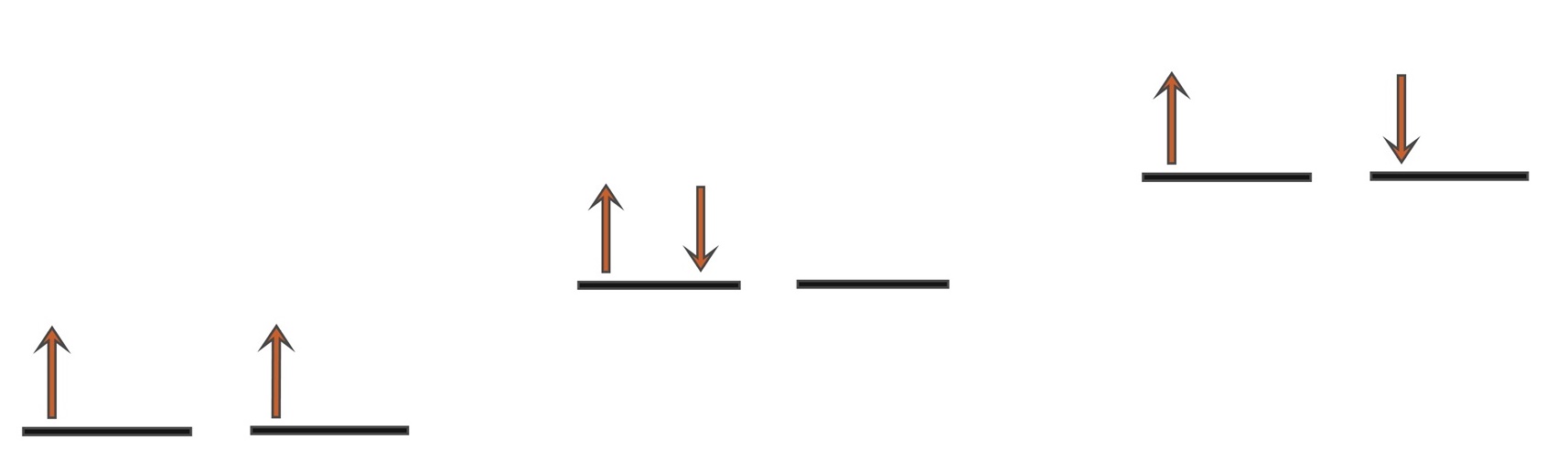
a) e-s parallel (triplet 3Σg), b) 2 e-s in 1 orbital (singlet 1Δg), c) e-s opposed (singlet 1Σg) Singlet oxygen is the name commonly used for the electronically excited state shown in b) and it is less stable than the normal triplet state a) by 94.7 kJmol-1. In isolation, singlet oxygen can persist for over an hour at room temperature. The other singlet state at 157.8 kJmol-1 shown in c) is very short lived and relaxes quickly to b). Because of differences in their electron shells, singlet and triplet oxygen differ in their chemical properties. Singlet oxygen is highly reactive.
Reactions of triplet dioxygen are restricted by conservation of spin state rules and at ambient temperatures this prevents direct reaction with all but the most reactive substrates, e.g. white phosphorus. At higher temperatures, or in the presence of suitable catalysts, reactions proceed more readily. For instance, most flammable substances are characterised by an autoignition temperature above which they will undergo combustion in air without an external flame or spark.
The energy difference between the ground state and singlet oxygen is 94.7 kJmol-1 which would correspond to a transition in the near-infrared at ~1263 nm. In the isolated molecule, this transition is strictly forbidden by spin, symmetry and parity selection rules, making it one of nature's most forbidden transitions. In other words, direct excitation of ground state oxygen by light to form singlet oxygen is very improbable. As a consequence, singlet oxygen in the gas phase is extremely long lived (72 minutes). Interaction with solvents however, can reduce the lifetime to microseconds or even nanoseconds.
Various methods for the production of singlet oxygen exist. A photochemical method involves the irradiation of normal oxygen gas in the presence of an organic dye as a sensitizer, such as methylene blue. Singlet oxygen can be produced chemically as well. One of the chemical methods is by the reaction of hydrogen peroxide with sodium hypochlorite. This is convenient in small laboratories and for demonstrative purposes:
H2O2 + NaOCl → O2(1Δg) + NaCl + H2O In photosynthesis, singlet oxygen can be produced from the light-harvesting chlorophyll molecules. One of the roles of carotenoids in photosynthetic systems is to prevent damage caused by any singlet oxygen that is produced by either removing excess light energy from chlorophyll molecules, or quenching the singlet oxygen molecules directly.
Biological considerations
Molecular dioxygen is a potentially strong oxidizing agent, based on its position in the electrochemical series. The standard redox potential is:
O2 + 4H+ + 4e- ⇄ 2 H2O E⦵ = +1.229 Vwhich is comparable to potassium dichromate (1.33 V). Nevertheless, reactions of dioxygen with most substrates tend to proceed very slowly at room temperature, in the gas phase or in solution. This relative inertness (unexpected considering it is a diradical) is critical for sustaining life in a dioxygen atmosphere. Perhaps one of the reasons for this stablilty is that the reaction above shows that a 4 electron change is required and this is highly improbable so that it is more likely that a sequence of steps involving 1 electron changes actually takes place.
O2 + e- → O2-* (superoxide)
O2-* + e- + 2H+ → H2O2 (peroxide)
H2O2 + e- + H+ → OH* (hydroxy radical)
OH* + e- + H+ → H2O (water)
The first step, that generates superoxide, has an adverse potential of -0.32 V (ΔG⦵= +30.9 kJmol-1) which imparts some kinetic stability to the molecule. Note that both dioxygen and superoxide ion are free radicals that exhibit paramagnetism. Species like superoxide, peroxide and the hydroxy radical are far too reactive to be allowed to accumulate in living systems and the primary defence of the cell appears to be to destroy them as soon as they are formed. This is done by a variety of enzymes including superoxide dismutase (SOD) and catalases. Parts of the immune system of higher organisms, however, create peroxide, superoxide, and singlet oxygen to destroy invading microbes. Reactive oxygen species also play an important role in the hypersensitive response of plants against pathogen attack.
Another issue with dioxygen is its low solubility in water at room temperature and atmospheric pressure. Dioxygen is more soluble in water than is dinitrogen. Water in equilibrium with air contains approximately 1 molecule of dissolved O2 for every 2 molecules of N2, despite the atmospheric ratio of approximately 1:4. The solubility of oxygen in water is temperature-dependent, and about twice as much (14.6 mgL-1) dissolves at 0 °C than at 20 °C (7.6 mgL-1). At 25 °C and 1 standard atmosphere (101.3 kPa) of air, freshwater contains about 6.04 milliliters (mL) of dioxygen per liter, whereas seawater contains about 4.95 mgL-1. At 5 °C the solubility increases to 9.0 mgL-1 (50% more than at 25 °C) for water and 7.2 mgL-1 (45% more) for sea water.
The oxygen in water is unavailable to mammals so that divers (and diving mammals such as whales and seals) are entirely dependent on the oxygen carried in the air in their lungs or their gas supply. For divers this is complicated since at higher partial pressures oxygen can cause acute toxicity leading to convulsions. Scuba (self-contained underwater breathing apparatus) divers using compressed air are restricted to diving above 30 m. If the diver descends to depths near 100 m they can become unconsciousness from nitrogen narcosis and death usually results. A dive to 30 m for 20 minutes puts the scuba diver at risk of nitrogen narcosis and decompression illness. By contrast, the elephant seal can dive to 1 km for 1 hour without risk of either condition.
Respiratory pigments are capable of fixing dioxygen from the atmosphere, transporting it to the reacting site and then releasing it. In addition they are able to counteract small fluctuations in supply or demand by storing dioxygen.
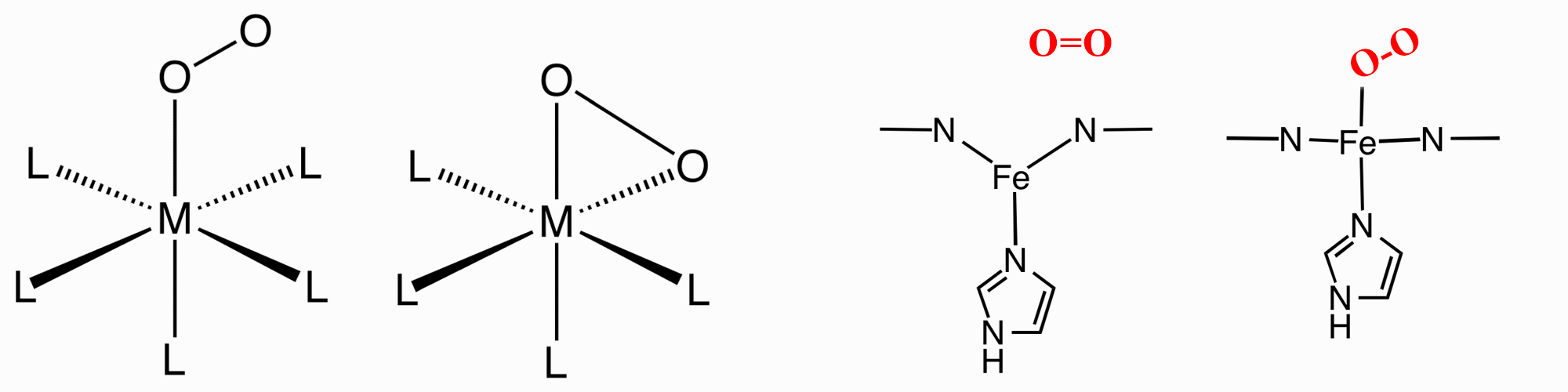 O2 can bind to a single metal center either "end-on" (η1-) or "side-on" (η2-). In Haemoglobin and Myoglobin it is "end-on". |
|
|
fully oxygenated Haemoglobin (with 4 Fe porphins and O2's highlighted) |
Molecular dioxygen, O2, is essential for cellular respiration in all aerobic organisms. Oxygen is used in mitochondria to help generate adenosine triphosphate (ATP) during oxidative phosphorylation. The reaction for aerobic respiration is essentially the reverse of photosynthesis and is simplified as:
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O ΔH= -2880 kJmol-1 In vertebrates, O2 diffuses through membranes in the lungs and into red blood cells. Haemoglobin binds O2, changing its colour from bluish red to bright red (CO2 is released from another part of haemoglobin through the Bohr effect). Other animals use haemocyanin (molluscs and some arthropods) or haemerythrin (spiders and lobsters). A liter of blood can dissolve 200 cm3 of O2.

