2.2: Self-Assessment- Bonding and Molecules + Answer
- Page ID
- 408596
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Problem #1
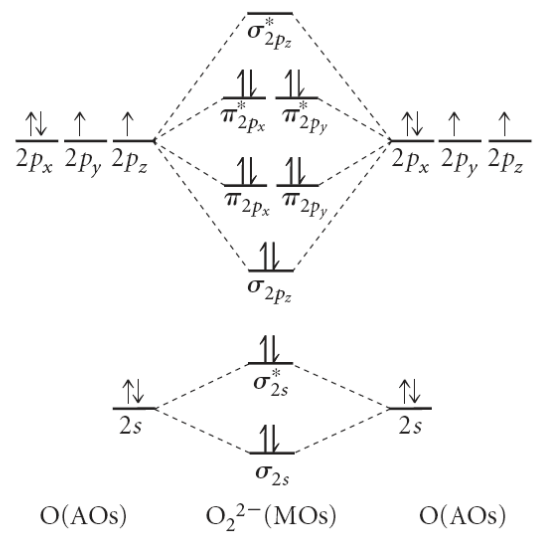
(a) Draw the energy level diagram that shows that the linear combination of atomic orbitals from two atoms of oxygen \((\mathrm{O})\) results in the formation of the stable molecule, \(\mathrm{O}_2{ }^{2-}\). The molecular orbitals in \(\mathrm{O}_2{ }^{2-}\) increase in energy according to the sequence \(\sigma_{2 s}, \sigma_{2 s} *, \sigma_{2 p_z}, \pi_{2 p_{x, y}}, \pi_{2 p_{x, y}}^*, \sigma_{2 p_z} *\).
- Answer
-
(b) Indium phosphide \((\operatorname{InP})\) is a semiconductor with a band gap, \(E_{\mathrm{g}}\), of \(1.27 \mathrm{eV}\). Calculate the value of the absorption edge of this material. Express your answer in meters.
- Answer
-
for absorption of incoming radiation, the following must be true:
\(E_{\text {radiation }}=E_g \text {. }\)
using the Planck relationship gives the wavelength of the absorption edge
\begin{aligned}
&E_{\text {radiation }}=\frac{h c}{\lambda} \\
&\therefore \lambda=\frac{h c}{E_g}=\frac{6.6 \times 10^{-34} \times 3 \times 10^8}{1.27 \times 1.6 \times 10^{-19}}=9.74 \times 10^{-7} \mathrm{~m} .
\end{aligned}
Problem #2
Chemical analysis of a silicon (Si) crystal reveals boron (B) at a level of 0.0003 atomic percent.
(a) Assuming that the concentration of thermally excited charge carriers from the \(\mathrm{Si}\) matrix is negligible, calculate the density of free charge carriers (carriers \(/ \mathrm{cm}^3\) ) in this \(\mathrm{Si}\) crystal.
- Answer
-
each B atom will attract an electron and thus create a "mobile hole"; we only have to determine the number of \(\mathrm{B}\) atoms \(/ \mathrm{cm}^3\) of \(\mathrm{Si}\). The atomic volume of the host crystal (\(\mathrm{Si}\)) is given on your \(\mathrm{PT}\) as \(12.05 \mathrm{~cm}^3 / \mathrm{mole}\).
\(\# \mathrm{Si}\) atoms \(/ \mathrm{cm}^3=\frac{6.02 \times 10^{23} \text { atoms }}{1 \text { mole }} \times \frac{1 \text { mole }}{12.05 \mathrm{~cm}^3}=5.00 \times 10^{22}\) atoms \(/ \mathrm{cm}^3\)
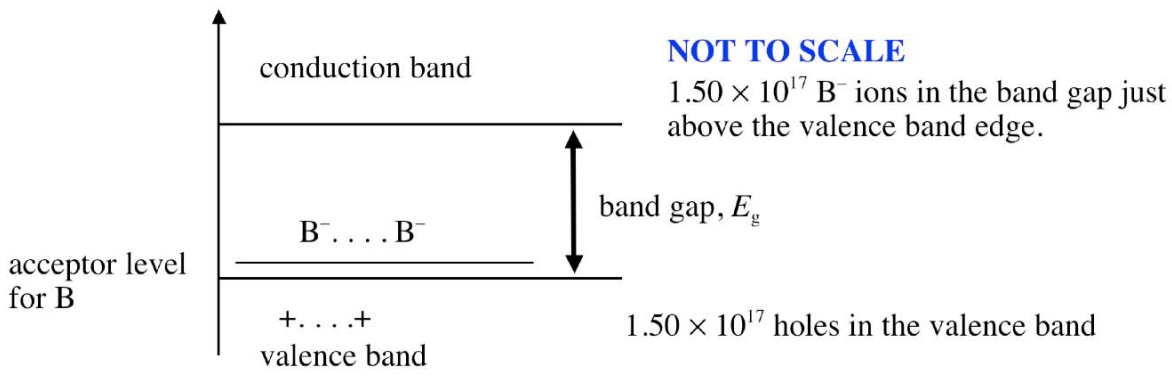
\(\therefore \# \mathrm{~B} \text{ atoms} / \mathrm{cm}^3=5.00 \times 10^{22} \times 0.0003 \times 10^{-2}=1.50 \times 10^{17} \mathrm{~B} / \mathrm{cm}^3\)
thus, the number of free charge carriers ("holes") is \(1.50 \times 10^{17} / \mathrm{cm}^3\); they are created through the acquisition of one electron by each \(\mathrm{B}\) atom from the valence band of the host $\mathrm{Si}$ crystal.
(b) Draw a schematic energy band diagram for this material and label the valence band, conduction band, band gap, and the energy level associated with the B impurity.
- Answer
-