2.1: Self-Assessment- Structure of the Atom + Answer
- Page ID
- 408592
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Problem #1
(a) Cerium has many isotopes (8 to be exact), but only \({}^{140}\mathrm{Ce}\) and \({}^{142}\mathrm{Ce}\) are present in substantial amounts. Which isotope of cerium is the most abundant?
- Answer
-
from the periodic table, you see that the atomic mass of \(\mathrm{Ce}\) is 140.115, which must be the weighted sum of the isotope masses here we assume that it is necessary to consider only \({ }^{140} \mathrm{Ce}\) and \({}^{142} \mathrm{Ce}\) so, \((x\) mass of \({ }^{140} \mathrm{Ce}\) \()+(1-x)\) mass of \({}^{142} \mathrm{Ce}\) \(=140.115\) for the purposes of this decision, we can approximate the atomic masses of the isotopes as \(\sim 140\) for \({ }^{140}\) and \(\sim 142\) for \({}^{142} \mathrm{Ce}\) and solve for \(\mathrm{x}\) \(\mathrm{x}=0.94\) which means that \({ }^{140} \mathrm{Ce}\) is the most abundant isotope of cerium
Production of chromium in an electric arc furnace would involve the reaction of carbon with chromium sesquioxide according to the following reaction:
\(\mathrm{Cr}_2 \mathrm{O}_3+a \mathrm{C}=b \mathrm{CO}+c \mathrm{Cr}\)
(i) Balance the equation, i.e., specify the values of \(a, b\), and \(c\). Insert the correct values below.
- Answer
-
\(\mathrm{Cr}_2 \mathrm{O}_3+3 \mathrm{C}=3 \mathrm{CO}+2 \mathrm{Cr}\)
(ii) Calculate the minimum amount of chromium (in \(\mathrm{kg}\) ) produced if the reaction consumed \(333 \mathrm{~kg} \mathrm{C}\) and produced the stoichiometric amount of Cr. Assume \(100 \%\) efficiency.
- Answer
-
\(333 \mathrm{~kg} \mathrm{C}=333000 / 12.011=27725\) moles \(\mathrm{C}\) the stoichiometric amount of \(\mathrm{Cr}\) is \(2 / 3\)
amount of carbon on a molar basis. Therefore, amount of \(\mathrm{Cr}=27725 \times 2 / 3\) moles of \(\mathrm{Cr} =(27725 \times 2 / 3) \times 51.996=961 \mathrm{~kg} \mathrm{Cr}\)
Problem #2
(a) Antimony has two isotopes, \({ }^{121} \mathrm{Sb}\) and \({ }^{123} \mathrm{Sb}\). Which isotope has the higher natural abundance?
- Answer
-
from the periodic table, you see that the atomic mass of \(\mathrm{Sb}\) is \(121.757\), which must be the weighted sum of the isotope masses
so, \(\left(x\right.\) mass of \(\left.{ }^{121} \mathrm{Sb}\right)+(1-\mathrm{x})\) mass of \({ }^{123} \mathrm{Sb}=121.757\)
for the purposes of this decision, we can approximate the atomic masses of the isotopes as \(\sim 121\) for \({ }^{121} \mathrm{Sb}\) and \(\sim 123\) for \({ }^{123} \mathrm{Sb}\) and solve for \(\mathrm{x}\)
\(\mathrm{x}=0.62\) which means that \({ }^{121} \mathrm{Sb}\) is the more abundant isotope of antimony
(b) Production of hafnium by the Kroll Process would involve the reaction of magnesium with hafnium tetrachloride according to the following reaction:
\(\mathrm{HfCl}_4+a \mathrm{Mg}=b \mathrm{MgCl}_2+c \mathrm{Hf}\)
(i) Balance the equation, i.e., specify the values of \(a, b\), and \(c\). Insert the correct values below.
- Answer
-
\(\mathrm{HfCl}_4+2 \mathrm{Mg}^2 \quad 2 \mathrm{MgCl}_2+\mathrm{Hf}\)
(ii) Calculate the minimum amount of magnesium (in \(\mathrm{kg}\) ) needed to convert \(111 \mathrm{~kg} \mathrm{HfCl}_4\) into elemental hafnium.
- Answer
-
\(111 \mathrm{~kg} \mathrm{HfCl}_4 = 111000/[178.49 + (4 \times 35.45)] = 347 \text{ moles } \mathrm{HfCl}_4\)
the stoichiometric amount of \(\mathrm{Mg}\) is twice the amount of \(\mathrm{HfCl}_4\) on a molar basis
\(\therefore\) amount of \(\mathrm{Mg}=347 \times 2\) moles of \(\mathrm{Mg}=(347 \times 2) \times 24.305=16.9 \mathrm{~kg} \mathrm{Mg}\)
Problem #3
(a) Show by means of a calculation that blue light of wavelength, \(\lambda=444 \mathrm{~nm}\), is not capable of exciting electrons in \(\mathrm{Li}^{2+}(\mathrm{g})\) from the state \(n=2\) to \(n=4\).
- Answer
-
let's equate the energy required to excite electrons in \(\mathrm{Li}^{2+}(\mathrm{g})\) from the state \(n=2\) to \(n=4\) with the minimum energy needed from an incident photon to cause the excitation
\begin{aligned}
\frac{h c}{\lambda} &=K Z^2\left(\frac{1}{n_f^2}-\frac{1}{n_i^2}\right) \\
\therefore \lambda &=\frac{h c}{K Z^2\left(\frac{1}{n_f^2}-\frac{1}{n_i^2}\right)}=\frac{6.6 \times 10^{-34} \times 3.00 \times 10^8}{2.18 \times 10^{-18} \times 3^2\left(\frac{1}{2^2}-\frac{1}{4^2}\right)} \\
&=5.38 \times 10^{-8} \mathrm{~m}=53.8 \mathrm{~nm}<444 \mathrm{~nm}
\end{aligned}\(\therefore\) since \(\mathrm{E}\) scales with \(1 / \lambda\), blue light of wavelength \(\lambda=444 \mathrm{~nm}\) does not have enough energy per photon to cause the excitation
(b) Is the value of the energy of transition from the state \(n=2\) to \(n=4\) in \(\mathrm{Li}^{2+}, \Delta E_{2 \rightarrow 4}\), greater than or less than the value of the energy of transition from the state \(n=1\) to \(n=2\) in \(\mathrm{Li}^{2+}\), \(\Delta E_{1 \rightarrow 2}\)? Explain with the use of an energy level diagram. There is no need to calculate the values of the two quantities.
- Answer
-
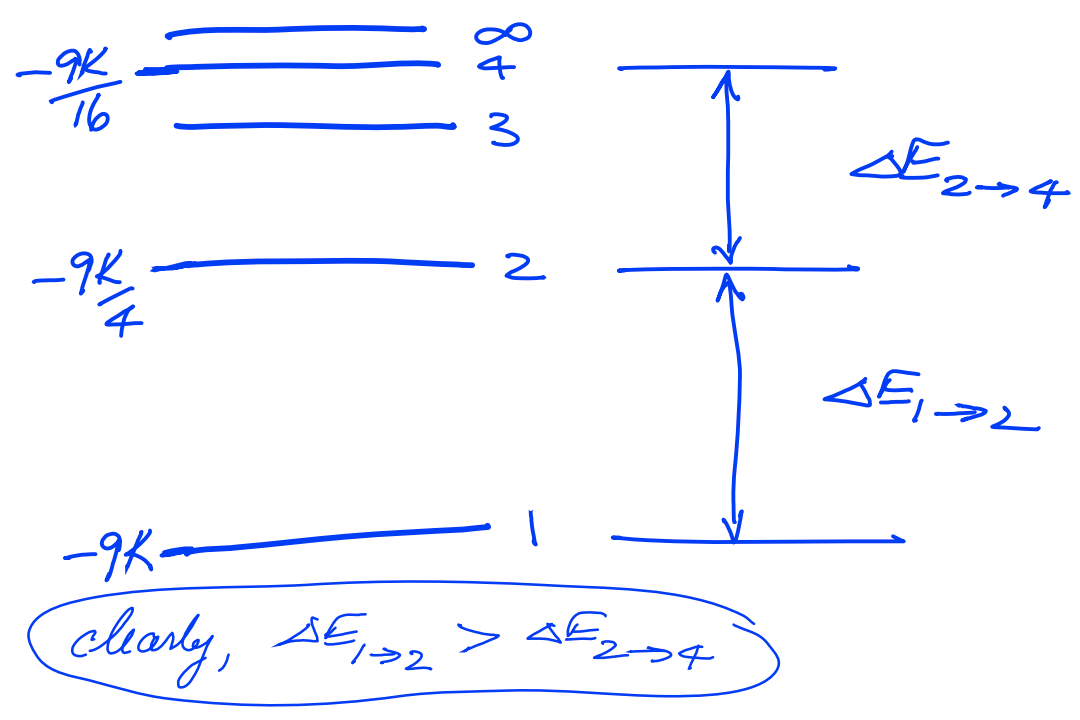
Problem #4
(a) In a gas discharge tube what is the minimum frequency \((v)\) of a photon capable of ionizing ground-state electrons in \(\mathrm{Li}^{2+}\)?
- Answer
-
here is the central concept: the energy of the incident photon must be at least as great as the ionization energy (I.E.)
\(\mathrm{Li}^{2+}\) is a one-electron atom, so we can calculate the I.E. using the Bohr Model
\(I . E .=E_{\infty}-E_1=0-\left(-\dfrac{K Z^2}{n^2}\right)\), where \(Z=3\) and \(n=1\) for the ground-state of \(\mathrm{Li}^{2+}\) and \(\mathrm{K}\) is the ground-state energy of atomic hydrogen
energy of incident photon is given by \(\mathrm{E}=\mathrm{h} v\)
\(v=\dfrac{K Z^2}{h} \dfrac{\left(2.18 \times 10^{-18} J\right)(3)^2}{6.6 \times 10^{-34}}=2.97 \times 10^{16} \mathrm{~Hz}\)
(b) Explain with reference to the relevant physical forces why the value of the \(1^{\text {st }}\) ionization energy of $\mathrm{Li}$ is less than the \(3^{\text {rd }}\) ionization energy of \(\mathrm{Li}\).
- Answer
-
the \(1^{\text {st }}\) ionization represents the removal of one of the 3 electrons from neutral \(\mathrm{Li}\)
the \(3^{\text {rd }}\) ionization represents the removal of the single electron from the \(\mathrm{Li}^{2+}\) ion
in the second case, the single electron alone feels the pull of the positive charge of the nucleus
in the first case the same positive charge is felt by three electrons; hence, each electron feels a weaker pull than is the case with a lone electron under the influence of the same positive charge


