6.4: Lewis Concept and Frontier Orbitals
- Page ID
- 151390
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Lewis acid-base concept generalizes the Brønsted and solvent system acid base concepts by describing acid-base reactions in terms of the donation and acceptance of an electron pair.
Under the Lewis definition
- Lewis acids are electron pair acceptors
- Lewis bases are electron pair donors
and in Lewis acid-base reactions, a Lewis base donates an electron pair to the Lewis acid, which accepts it. The reaction between borane, \(BH_3\), and \(NH_3\) is the classic example:

In this case the Lewis acid-base reaction results in the formation of a bond between \(BH_3\), and \(NH_3\). When the acid and base combine to form a larger unit, that unit is said to be an adduct, and the resulting bond is said to be a coordinate covalent or dative bond. Such coordinate covalent bonds are often represented by an arrow that indicates the direction of electron donation from the base to the acid. For instance, the reaction between \(BH_3\) and \(NH_3\) could also have been written as
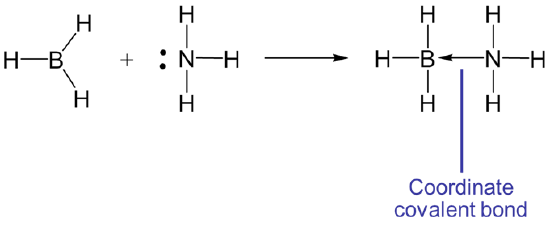
The arrow notation for the coordinate covalent bond is really just a convenient formalism - a bookkeeping tool to help keep track of where the electrons came from and where they might return if the reverse reaction occurs. In actuality a coordinate covalent bond is just an ordinary covalent bond like any other.
For example, in Brønsted acid-base reactions, the hydrogen ion is an acid because it accepts an electron pair from the Brønsted base. Consequently, under the Lewis acid-base concept, Brønsted acid-base reactions involve the formation of an adduct between \(H^+\) and a base.
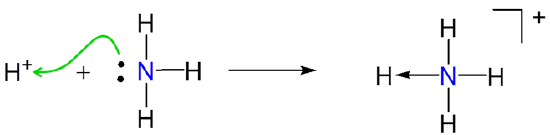 \[ \nonumber \]
\[ \nonumber \]
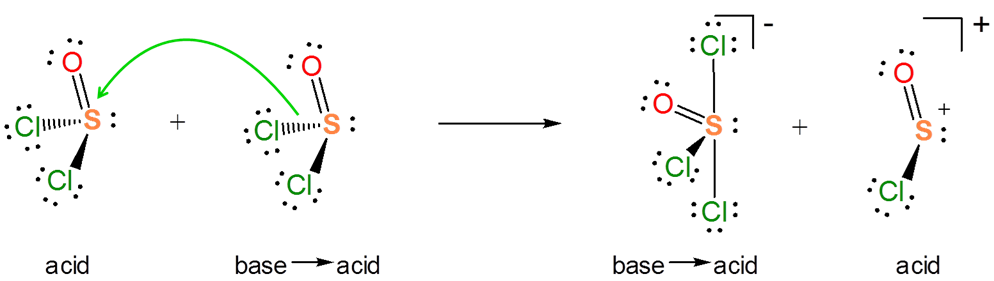
The Lewis acid-base concept nicely explains ionization reactions involving nonaqueous solvents. For instance, the autoionization of \(SOCl_2\) is an acid-base reaction between two \(SOCl_2\) molecules.
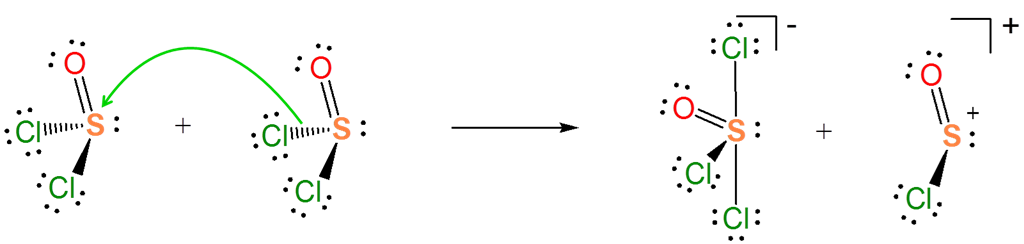 \[ \nonumber \]
\[ \nonumber \]
Explain how the autoionization of \(SOCl_2\) is a Lewis acid-base displacement reaction.
Solution
In the autoionization of \(SOCl_2\) the pair of electrons donated comes from the S-Cl bond, and the S-Cl bond is broken to give a lone pair bearing a \(Cl^-\) base and an \(SOCl^+\) Lewis acid fragment. If you are having trouble seeing how this works, it can be instructive to consider the reverse of this process. It is a Lewis acid-base reaction to give a Lewis acid-base adduct:
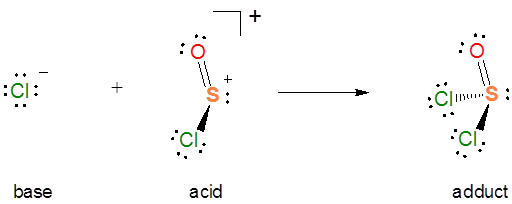
From the autoionization reaction it is also apparent that \(SOCl_2\) itself acts as a Lewis acid towards the liberated \(Cl^-\). So the autoionization reaction involves a transfer of the \(Cl^-\) base between the two Lewis acids - a Lewis acid-base displacement reaction.
It can be helpful to keep several distinctions in mind when using the Lewis acid-base concept to describe chemical reactions.
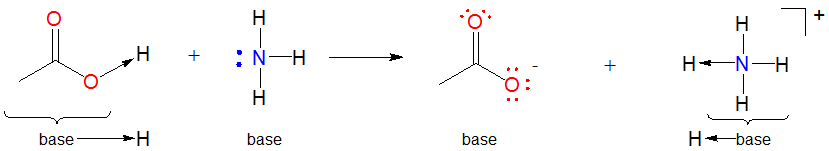
1. Many Lewis-Acid base reactions are displacement reactions. This is because the hydrogen ion is usually bound to something at the start of the reaction. In such cases the Lewis acid \(H^+\) unit is transferred from one Lewis base to another :
 \[ \nonumber \]
\[ \nonumber \]
Such acid-base reactions are sometimes called displacement reactions since the base group in the initial Lewis acid-base complex is displaced by the incoming Lewis base to generate another complex.
2. Substances are sometimes considered amphoteric because they exhibit Lewis acidity and basicity at different types of atomic centers. The classic example is aluminum hydroxide, \(\ce{Al(OH)3}\). In water \(\ce{Al(OH)3}\) can act as a Lewis acid towards OH- ion. The reaction occurs by formation of an adduct at \(\ce{Al(OH)3}\)'s Al3+ center:
\[\ce{Al(OH)3 + OH^{-} \rightarrow Al(OH)4^{-}} \nonumber \]
Notice that in addition to acting as a Lewis acid, in this reaction \(Al(OH)_3\)
- does not act as a Brønsted acid or base since no H+ ion transfer occurs
- but does act as an Arrhenius acid since OH- ion is consumed from solution, decreasing [OH-] and increasing [H+]
In water \(\ce{Al(OH)_3}\) also acts as a Lewis base towards H+ through the lone pairs on its hydroxide ligands.
\[\ce{Al(OH)3 + 3 H^{+} + 3 H2O \rightarrow Al(H2O)6^{3+}} \nonumber \]
Notice that in addition to acting as a Lewis base, in this reaction \(\ce{Al(OH)3}\) also acts as a
- Brønsted base, since a H+ ion is transferred onto the OH- ligand
- Arrhenius base, since H+ ion is consumed from solution, decreasing [H+]
- Lewis acid at its Al3+ center, since Al-O metal-ligand bonds are formed
Substances that rapidly undergo Lewis acid-base reactions are called nucleophiles and electrophiles.
Two factors govern the course of chemical reactions - thermodynamics and kinetics. Thermodyamics determines what possible fates of the reaction can take place while kinetics determines which among those possible fates will take place quickly under a particular set of reaction conditions. Because of this, it can be helpful to distinguish Lewis acids and bases that tend to undergo reaction quickly with one another from those which do so more slowly. For this reason, synthetic chemists use the terms electrophile and nucleophile to refer to Lewis acids and bases that react quickly:
- electrophile - Lewis acids that rapidly react with a given Lewis base or class of Lewis bases are said to be good electrophiles
- nucleophile - Lewis bases that rapidly react with a given Lewis acid or class of Lewis acids are said to be good nucleophiles
The electrophile-nucleophile concept is sometimes referred to as the Ingold-Robinson acid-base concept after two organic chemists who did much to illustrate the utility of thinking about Lewis acid-base reactions in kinetic terms.
Several distinctions that should be kept in mind when using the electrophile-nucleophile/Ingold-Robinson concept to think about chemical reactivity:
1. Whether a given Lewis acid or base is able to react rapidly depends on the reaction conditions and the substrate (substance it is reacting with). This means that in principle, the terms nucleophile and electrophile should not be used without appropriately qualifying what the substances act as a nucleophile or electrophile towards and under what conditions. Unfortunately this is rarely done. In such cases, the conditions and substrate must be inferred from the context. For instance, when you learned that the chloride ion is a good nucleophile when studying organic chemistry, what was meant is that chloride is a good nucleophile towards electrophilic carbon atoms in organic molecules.
2. Acid and base strength and electrophilicity-nucleophilicity are not exactly the same thing. In many contexts where Lewis acids and bases are classified as electrophilic and nucleophilic substances, it is common to also refer to substances as being good acids or bases on the basis of their Brønsted acidity and basicity. In these contexts, the terms acid and base typically refer to the thermodynamic propensity of a substance to give and accept hydrogen ions while the terms nucleophile and electrophile refer to a substance's kinetic propensity to react with carbon-based Lewis acids and bases, respectively.
A substance may be both a good nucleophile and a strong Brønsted base. For instance, alkyllithium carbanion reagents and alkali metal hydrides are strong bases and good nucleophiles towards electrophilic carbon atoms in organic solvents at moderate temperatures. However, this is not always the case. Under such conditions iodide ion, \(I^-\), is a good nucleophile towards electrophilic carbon atoms but is a poor Brønsted base (it is the conjugate of the strong acid HI). In fact, for the halide ions the order of Brønsted basicity is the opposite of the order of nucleophilicity towards electrophilic carbon:
stronger Brønsted base: \(F^-\), \(Cl^-\), \(Br^-\), \(I^-\): weaker Brønsted base
better nucleophlie: \(I^-\), \(Br^-\), \(Cl^-\), \(F^-\): poorer nucleophile
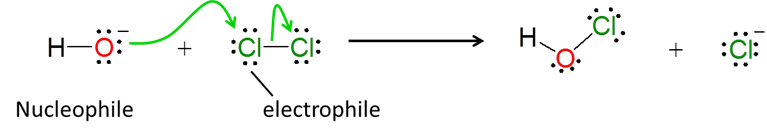
3. Although the nucleophile-electrophile concept is most often used to describe organic reactions, it is also useful for describing inorganic reactions as well. For instance, the formation of hypochlorite ion in chlorine water possibly involves nucleophilic attack of hydroxide on an electrophilic chlorine atom in an SN2 type process:
 \[ \nonumber \]
\[ \nonumber \]
The Usanovich Acid-base concept encompasses a wider range of reactions than the Lewis acid-base concept but is perhaps too general to serve as a convenient framework for understanding reaction chemistry. The Usanovich acid-base concept was developed by the Russian chemist Mikhail Usanovich and extends the Lewis acid-base concept's definition of acids and bases as electron pair donors and acceptors even further. Within the Usanovich definition:
- Acids are anything that accepts electrons, increases cation concentrations, decreases anion concentrations, or reacts with bases
- Bases are anything that donates electrons, increases anion concentrations, decreases cation concentrations, or reacts with acids
These definitions essentially expand the Lewis definition by removing the requirement that the electrons donated or accepted be a pair and practically mean that Usanovich acid-base reactions include Lewis acid-base reactions plus oxidation-reduction reactions. In other words:
- Usanovich acids are Lewis acids plus oxidants
- Usanovich bases are Lewis bases plus reductants
Under this definition \(BF_3\), \(Fe^{3+}\), and \([Fe(CN)_6]^{3-}\) all act as acids, and \(:NH_3\), \(CN^-\), and Zn are all bases:
\[\underset{\textcolor{red}{acid}}{BF_3} + \underset{\textcolor{blue}{base}}{NH_3} → F_3B-NH_3 \nonumber \]
\[\underset{\textcolor{red}{acid}}{Fe^{3+}} + 6 \underset{\textcolor{blue}{base}}{CN:^-} → [Fe(CN)_6]^{3-} \nonumber \]
\[2\underset{\textcolor{red}{acid}}{[Fe(CN)_6]^{3-}} + \underset{\textcolor{blue}{base}}{Zn} → 2[Fe(CN)_6]^{3-} + Zn^{2+} \nonumber \]
However, notice that while it is easy to think about how any acids might interchangeably react with a given base (and vice versa), under the other acid-base concepts chemists have found it more difficult to think about reactions between oxidants/reductants and Lewis acids and bases. For example, it is difficult to think about how a reaction between \(BF_3\) and Zn metal might occur. Would two Zn donate a pair of electrons to the \(BF_3\)? For this reason, most chemists find it more convenient to think about Lewis acid-base and redox reactions under separate categories rather than unite them under the Usanovich definition.
\[2\underset{\textcolor{red}{oxidant}}{[Fe(CN)_6]^{3-}} + \underset{\textcolor{blue}{reductant}}{Zn} → 2[Fe(CN)_6]^{3-} + Zn^{2+} \nonumber \]
Again, it can be helpful to remember Huheey's dictum that deciding between acid-base concepts is not a matter of which concept is the most correct but rather of which is more convenient for a given application.

