6.3.11: Non-nucleophilic Brønsted-Lowry Superbases
- Page ID
- 157373
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Non-Nucleophilic Organic Superbases Act as Strong Bases in Organic Solvents but Are Unreactive Towards Other Electrophiles
A variety of strong organic and inorganic bases are available for use in organic synthesis (alkyllithium reagents, diisopropyl amide derivatives, hydrides, and hydroxides). Some of these exhibit poor functional group tolerance owing to their ability to react with electrophilic functional groups. Hence there is considerable interest in the development of bases that can remove hydrogen ions from very weakly acidic organic substrates (i.e. like C-H bonds) without reacting with electrophilic functional groups. Classical non-nucleophilic bases used widely in organic chemistry include diisopropylethylamine (DIEA), lithium diisopropylamide (LDA), and the simple and complex ionic hydrides like \(\ce{CaH2^{+2}}\) and \(\ce{LiAlH4}\). The latter, however, are non-nucleophilic by virtue of their insolubility in organic solvents, on account of which they largely act as bases when an organic substrate interacts with the hydride at a crystal surface.
Many strong bases are called superbases in everyday usage. However, more rigorous definitions have been developed to clearly distinguish superbases from simple strong bases. Most of these use some aspect of the thermodynamics of hydrogen ion bonding by 1,8-Bis(dimethylamino)naphthalene as a delimiter between ordinary strong bases and superbases. In other words, by these criteria any base stronger than 1,8-bis(dimethylamino)naphthalene is considered a superbase. By this measure organic superbases may be defined as those having larger proton affinities than 1048 kJ/mol,1 although most workers use defined superbases as those having a \(pK_a\) greater than 1,8-bis(dimethylamino)naphthalene's value of 12.2
Non-nucleophilic Organic Superbases Use Charge Delocalization, Proton Chelation, or Both to Tightly Bind Hydrogen Ion
A variety of compounds with a high affinity for hydrogen ions in organic solvents have been specifically developed as strong non-nucleophilic organic superbases.3 Common classes include:
Napthalene-type proton sponges
Napthalene-type proton sponges are two basic groups held in close proximity to one another at the 1 and 8 positions of a napthalene ring. The compound 1,8-Bis(dimethylamino)naphthalene is perhaps the best known superbase of this type and is even sold by the trade name of Proton Sponge™. Its structure is shown below.
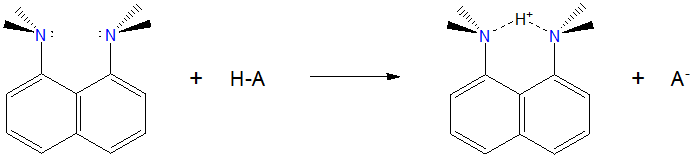
Proton sponge has the typical properties of an organic superbase. Unlike lithium diisopropylamindes and metal hydride reagents, 1,8-Bis(dimethylamino)naphthalene is only weakly basic in water. However, it possesses a very strong affinity for hydrogen ions in organic solvents.
In this case the high basicity of 1,8-Bis(dimethylamino)naphthalene is due to a combination of steric and electronic factors. First, it is a hydrogen chelator in that the hydrogen is held in a strong symmetric N---H---N hydrogen bond in its conjugate base. Second, the formation of that hydrogen bond relieves some of the steric strain associated with dimethyl amino groups held close to one another at the 1 and 8 positions on the naphthalene ring.
Aminidines and Guanidenes
Aminidines are nitrogen derivatives of carboxylic acid derivatives. Acyclic aminidines have \(pK_a\) values ~12, similar to that of 1,8-Bis(dimethylamino)naphthalene, although cyclic aminidines are several orders of magnitude higher. For example, DBU is estimated to exhibit a \(pK_a\) value of 24.3 in MeCN.4 Examples of aminidine superbases include the organic catalysts 1,8-Diazabicyclo(5.4.0)undec-7-ene (DBU) and 1,5-Diazabicyclo(4.3.0)non-5-ene (DBN), and vinamidinium superbases, which bind hydrogen ion at the imine nitrogen.
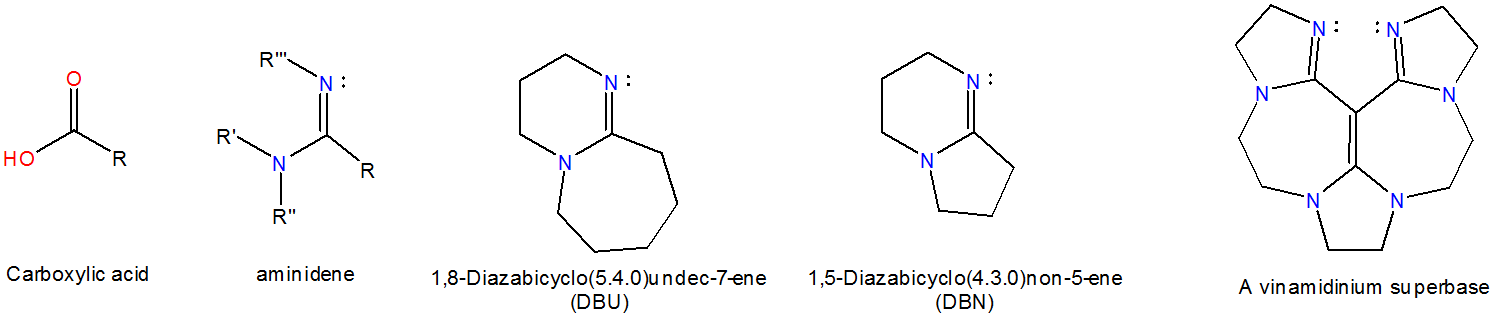
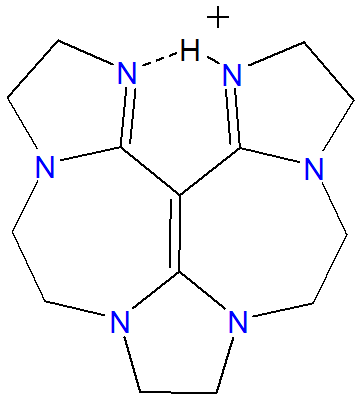
Of these the vinamidinium superbases are hydrogen ion chelators.
Guanidenes are nitrogen analogues of carbonate and bind hydrogen ion at a nitrogen lone pair just as aminidines do, although monomeric guanidines are slightly more basic (\(pK_a ~13\)) than analogous aminidines ( \(pK_a ~12\)). Common classes of guanidene superbases include pentalkyl and bicyclic guanidines, shown below.
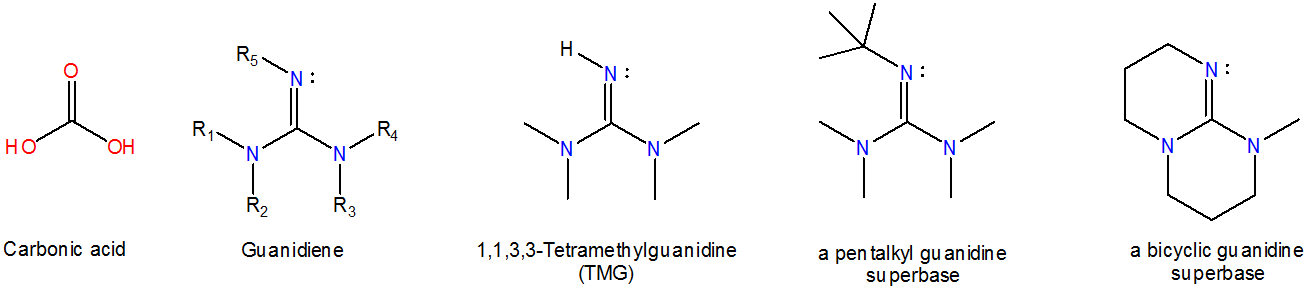
The high basicity of aminidines and guanidines is derived from the ability of these systems to delocalize charge in the protonated form.
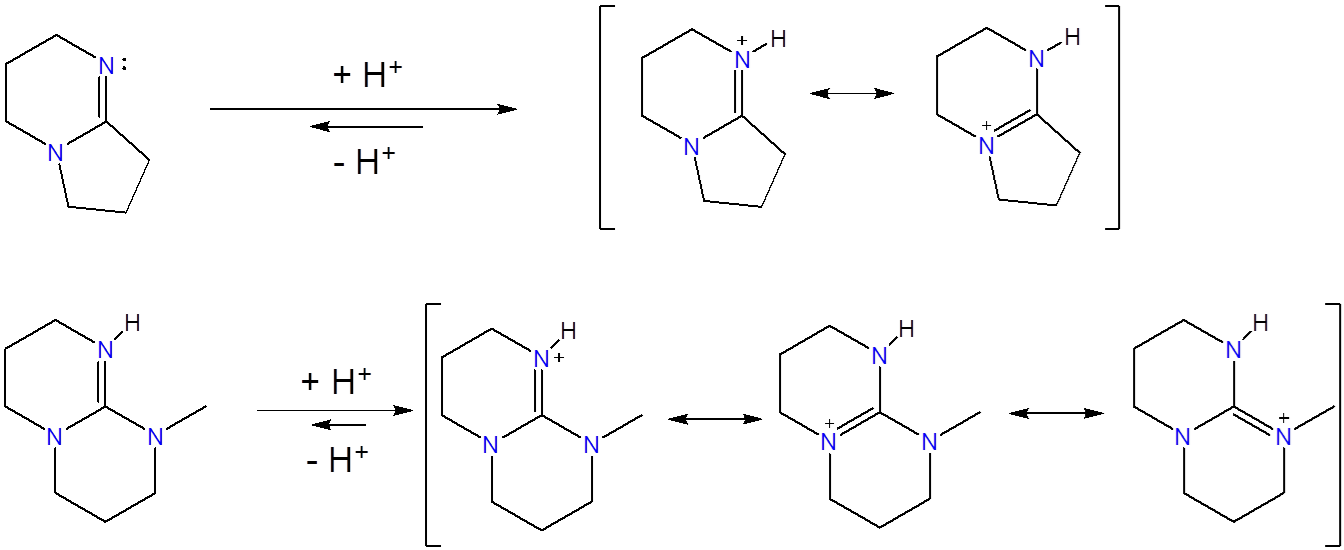
Use the structure of protonated aminidines and guanidines to explain why guanidines are stronger bases than their aminidine analogues.
- Answer
-
There is greater delocalization of charge in guanidinium ions. According to the resonance picture of bonding, in guanidinium ions the positive charge is delocalized over three nitrogen atoms while in amidinium ions it is only delocalized over two.
Phosphatrane-type Superbases
These exhibit enhanced basicity at the phosphorus atom of a special type of azaphosphine called a proazaphosphatrane:
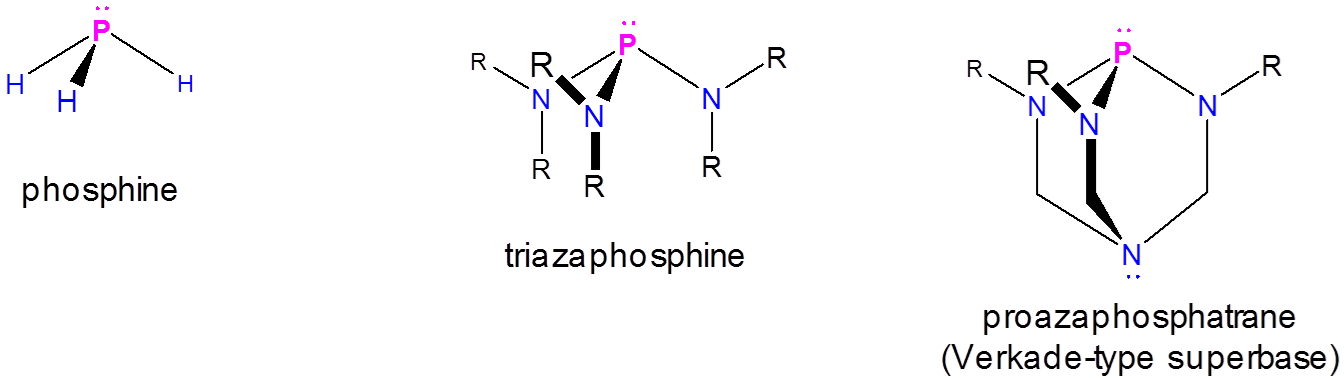
Of these the best known is Verkade's superbase, which is estimated to have a \(pK_a\) of 29 in acetonitrile,5 meaning it is considerably more basic than aminidine and guanidine type superbases. The key to the remarkable basicity of Verkade's superbase is the ability of the proazaphosphoatrane's amine to stabilize the protonated azaphosphine through the formation of a P-N bond, giving a pseudo trigonal bipyramidal phosphorous.
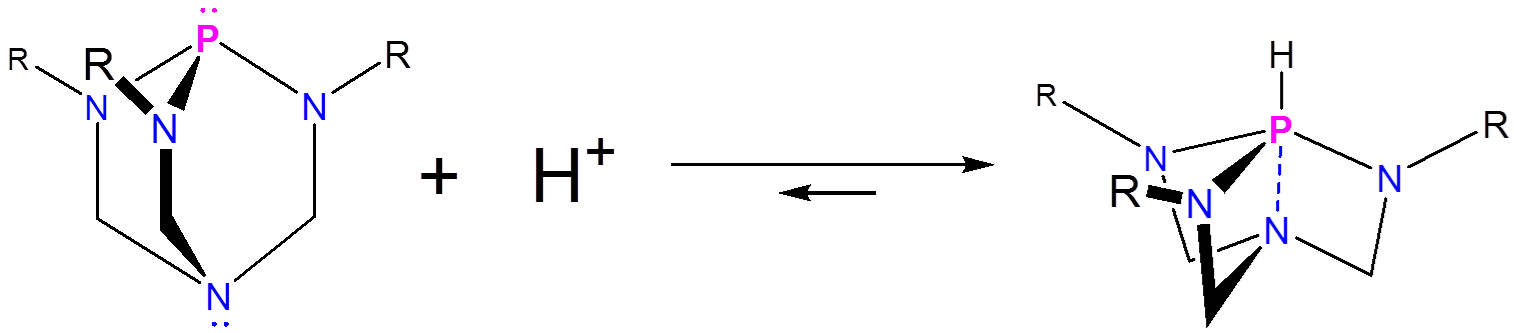
These interactions are so stabilizing that the transannular amine nitrogen exhibit no basicity, even when superacids like Magic Acid are added.6
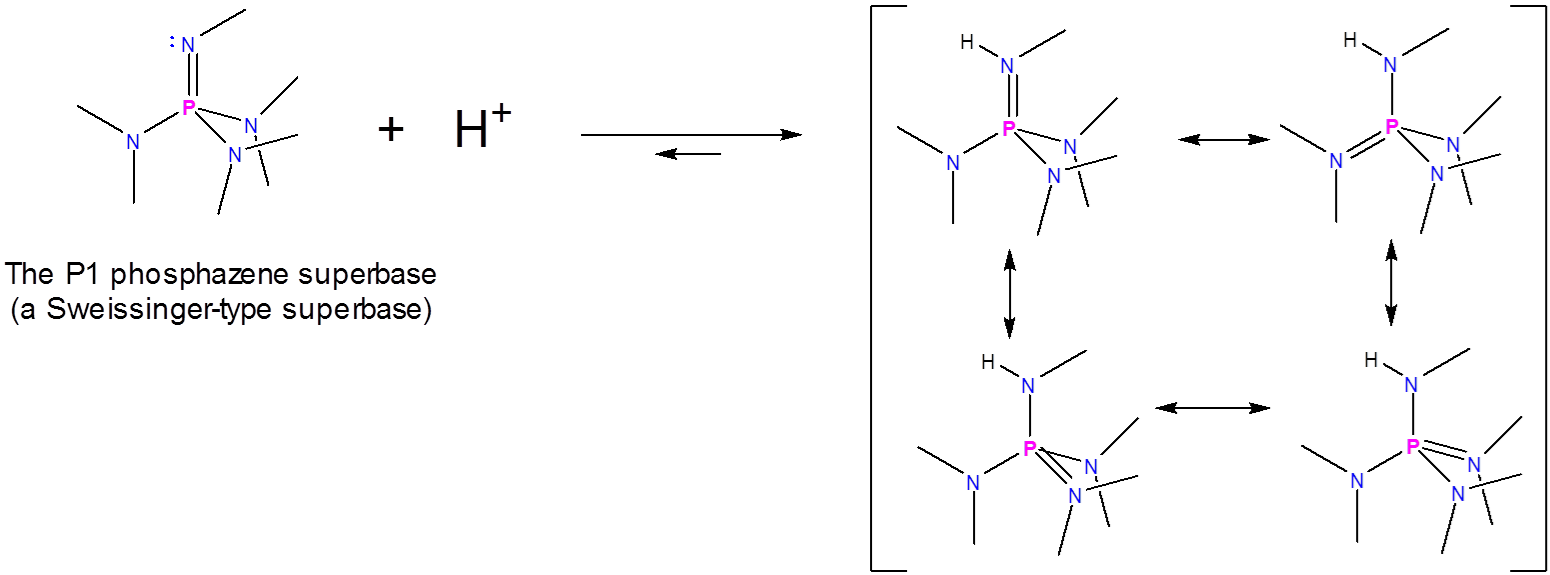
Phosphazene Superbases
Phosphazene superbases, also known as Schwesinger superbases, function similarly to aminidines and guanidines in that they bind a hydrogen ion at an imine nitrogen to give a resonance-stabilized cation. It is just that in this case the imine is that of a phosphazene and the greater resonance stabilization of the resulting cation means that they exhibit greater basicity. One phosphazene superbase has been reported to have a \(pK_a\) of 42 in acetonitrile.
References
- Gal, J.‐F.; Maria; P.‐C.; Raczynska, E. D. Thermochemical aspects of proton transfer in the gas phase. Journal of Mass Spectrometry, 2001; 36: 699–716
- The \(pK_a\) of 1,8-Bis(dimethylamino)naphthalene is taken from Benoit, R. L.; Lefebvre, D.; Fréchette, M., Basicity of 1,8-bis(dimethylamino)naphthalene and 1,4-diazabicyclo[2.2.2]octane in water and dimethylsulfoxide. Canadian Journal of Chemistry 1987, 65 (5), 996-1001.
- Ishikawa, T. Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts; Wiley: New York, 2009.
- Unless otherwise noted \(pK_a\) values other than that reported for 1,8-Bis(dimethylamino)naphthalene are taken from Kaupmees, K.; Trummal, A.; Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta. 87: 385–395. doi:10.5562/cca2472
- Kovačević, B.; Barić, D.; Maksić, Z. B., Basicity of exceedingly strong non-ionic organic bases in acetonitrile —Verkade's superbase and some related phosphazenes. New Journal of Chemistry 2004, 28 (2), 284-288.
- Akiba, K-Y. Organic Main Group Chemistry; Wiley: New York, 2011.

