7.9: Comparison Between Silicon and Carbon
- Page ID
- 212898
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)An understanding of the differences between carbon and silicon is important in understanding the relative chemistry of these Group 14 elements.
Size
As expected silicon is larger than carbon due to the presence of a second shell: i.e., C = 1s2 2s2 2p2 while Si = 1s2 2s2 2p6 3s2 3p2. A comparison of the relative sizes of carbon and silicon are given in Table \(\PageIndex{1}\).
| Element | Atomic radius (Å) | Covalent radius sp3 (Å) | van der Waal radius (Å) |
| C | 0.70 | 0.75 | 1.70 |
| Si | 1.10 | 1.14 | 2.10 |
Covalent and van der Waal radii from Royal Society of Chemistry Online Periodic Table. Webelements has a more detailed discussion of all three types of radii, the atomic radii quoted here are empirical.
Coordination number
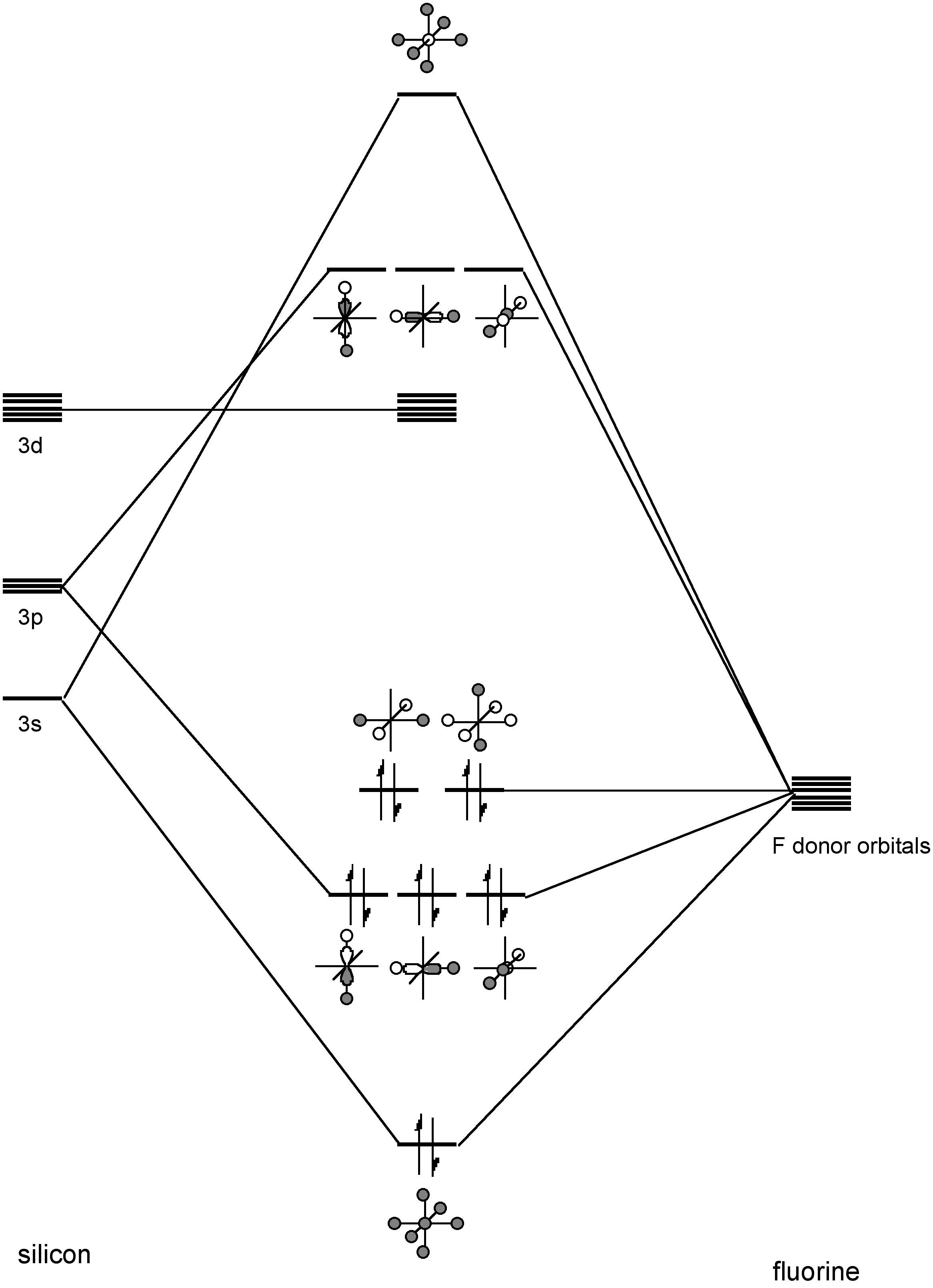
Carbon is known to have a coordination number of 2, 3, and 4 in its compounds depending on the hybridization. A coordination number of 1 can also be considered for CO and CN-. Four-coordinate carbon may be considered to be coordinatively saturated. In contrast, in the absence of overwhelming steric bulk, silicon is observed to have coordination numbers of 3, 4, 5, and 6. Examples of five and six-coordinate silicon include Si(acac)2Cl and SiF62-, respectively. Coordination numbers of higher than 4 have been ascribed to the use of low-lying d orbitals; however, calculations show these are not significant. Instead, hypervalent silicon is better described by the formation of 3-center molecular orbitals, e.g., Figure \(\PageIndex{1}\).
Note
A hypervalent molecule is a molecule that contains one or more typical elements (Group 1, 2, 13-18) formally bearing more than eight electrons in their valence shells.
Electronegativity
The electronegativities of silicon and carbon are given in Table along with hydrogen. Since carbon is more electronegative than hydrogen the C-H bond is polarized towards carbon resulting in a more protic hydrogen (Figure \(\PageIndex{2}\)a). In contrast, the lower electronegativity of silicon results in a more hydridic hydrogen (Figure \(\PageIndex{2}\)b). This difference is reflected in the reaction chemistry of SiH4 versus CH4.
| Element | Pauling scale |
| C | 2.55 |
| H | 2.20 |
| Si | 1.90 |
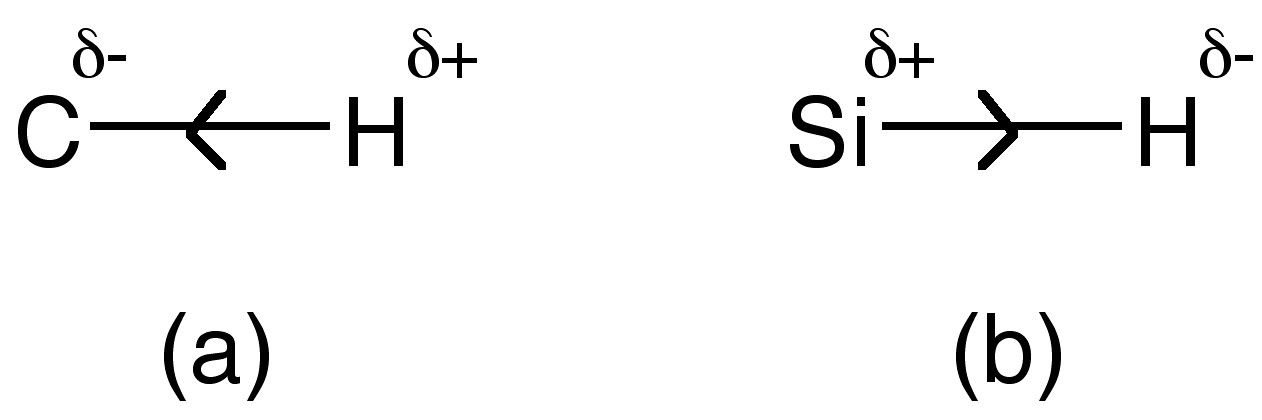
Bond energies
The E-E and E-O bond energies for carbon and silicon are given in Table \(\PageIndex{3}\). The bond energy for a C-C bond is slightly greater than for a C-O bond, while the Si-O bond is significantly stronger than the Si-Si bond. This difference is reflected in the chemistry of silicon versus carbon compounds. The chemistry of carbon is dominated by catenation: the ability of a chemical element to form a long chain-like structure via a series of covalent bonds. Although silicon does form Si-Si bonds, they are far more reactive than their C-C analogs, and polymers of silicon are predominantly comprised of Si-O chains (as a result of the very strong bond).
| Element | E-E bond energy (kJ/mol) | E-O bond energy (kJ/mol) |
| C | 345.6 | 357.7 |
| Si | 222 | 462 |
Multiple bonds
While unsaturated compounds for carbon (i.e., alkenes and alkynes) are common, the analogous silicon compounds (disilenes) were only reported in 1981, and disilynes in 2004. The Si=Si double bond lengths are 2.14 - 2.29 Å which is 5 - 10% shorter than the Si-Si single bond lengths. This bond shortening is less than ca. 13% in carbon compounds.
Note
The traditional lack of multiple bonds for the Period 3 elements and lower led to the formulation of the double bond rule which states that chemical elements with a principal quantum number greater than 2 do not form multiple bonds (e.g., double bonds and triple bonds) with themselves or with other elements. This rule was made obsolete starting from 1981 with the discovery of silicon and phosphorus double bonds. Double bonds that would ordinarily not form can be stabilized with proper functional groups through kinetic stabilization, i.e., either electronically or sterically.
Bibliography
- R. West, M. J. Fink, and J. Michl, Science, 1981, 214, 1343.
- A. Sekiguchi, R. Kinjo, and M. Ichinohe, Science, 2004, 305, 1755.

