7.8: Carbon Halides
- Page ID
- 212897
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There are two general classes of carbon halides.
- Homoleptic halides, e.g., CCl4, CCl2F2, C6Cl6, etc.
- Carbonyl halides, e.g., Cl2C=O.
A summary of some simple carbon halides is given in Table \(\PageIndex{1}\).
| Compound | Mp (°C) | Bp (°C) | Remarks |
| CF4 | -185 | -128 | Very stable gas |
| CCl4 | -23 | 76 | Colorless liquid, stable |
| CBr4 | 93 | 190 | Pale yellow solid, decomposes upon boiling |
| CI4 | 171 | - | Bright red solid, decomposes prior to boiling, sublimed at low pressure |
| F2C=O | -114 | -83 | Decomposed by H2O |
| Cl2C=O | -118 | 8 | Phosgene, highly toxic |
| Br2C=O | - | 65 | Fumes in air |
Carbon tetrahalides
The carbon tetrahalides are generally prepared by the direct (thermal) reaction of carbon with the appropriate halogen, (7.8.1); however, specific syntheses are possible for each derivative.
\[ \text{C + 2 X}_2 \rightarrow \text{CX}_4\]
In addition to the direct reaction of fluorine with carbon, CF4 can be prepared from SiC, (7.8.2). The SiF4 side product is removed by passing the reaction mixture through NaOH solution, in which SiF4 reacts to form silicate. The difference in reactivity of SiF4 and CF4 is attributable to the lack of an energetically accessible five-coordinate intermediate required for the associative mechanism.
\[ \text{SiC + F}_2 \rightarrow \text{SiF}_4\text{ + CF}_4 \]
Carbon tetrabromide can be obtained by bromination of CH4 with HBr or Br2, or by the reaction of CCl4 with AlBr3, (7.8.3). Carbon tetraiodide (CI4) can be made by the Lewis acid catalyzed halogen exchange reaction, (7.8.4).
\[ \text{3 CCl}_4\text{ + 4 ALBr}_3 \rightarrow \text{3 CBr}_4\text{ + 4 AlCl}_3\]
\[ \text{CCl}_4\text{ + 4 C}_2\text{H}_5\text{I} \rightarrow \text{CI}_4 \text{ + 4 C}_2\text{H}_5\text{Cl}\]
CF4 is very stable. In fact, it is so stable that it does not even react with molten sodium. In contrast to CF4, carbon tetrachloride (CCl4) reacts readily with alkali metals (K and Na) or other strong reducing agents (e.g., F2, Al, Ba, Be, and Zn). While CCl4 is thermodynamically unstable with respect to hydrolysis, it is kinetically stable, and thus finds extensive use as a solvent. Photolysis can result in the transfer of a chloride radical to various substrates. It is also used in the conversion of metal oxides to the chlorides. Carbon tetrabromide (CBr4) is insoluble in water and other polar solvents, but soluble in benzene. Carbon tetraiodide (CI4) decomposes thermally, (7.8.5).
\[ \text{2 CI}_4 \rightarrow \text{2 I}_2 \text{ + I}_2\text{C=CI}_2 \]
The decreasing stability of CX4, from fluorine to iodine, is directly related to the C-X bond energy.
| C-X | Bond energy (kJ/mol) |
| C-F | 485 |
| C-Cl | 327 |
| C-Br | 285 |
| C-I | 213 |
Hazards
Despite its use as a solvent CCl4 has significant hazardous effects. Inhalation of carbon tetrachloride vapor can cause headaches, mental confusion, depression, fatigue, loss of appetite, nausea, vomiting, and coma. The symptoms can take many hours to appear. The vapor and liquid irritate the eyes, and internal irritation, nausea, and vomiting are caused when taken orally. Chronic effects from prolonged inhalation include bronchitis and jaundice, while skin exposure can cause dermatitis.
Carbon tetrabromide is toxic by inhalation, and the vapor is narcotic if taken in high concentrations. As with CCl4, CBr4 can react explosively with alkali metals.
Higher homoleptic halides
Organic compounds that contain only carbon and a halogen are called halocarbons, and these include fluorocarbons and chlorocarbons. The easiest route to fluorocarbons involves the reaction of a hydrocarbon with a high valent fluoride (e.g., CoF3) or the reaction of a chlorocarbon with SbF3. In general, chlorocarbons with sp3 carbon atoms are more stable than those with sp2 carbon centers. The exception to this is aromatic compounds such as C6Cl6.
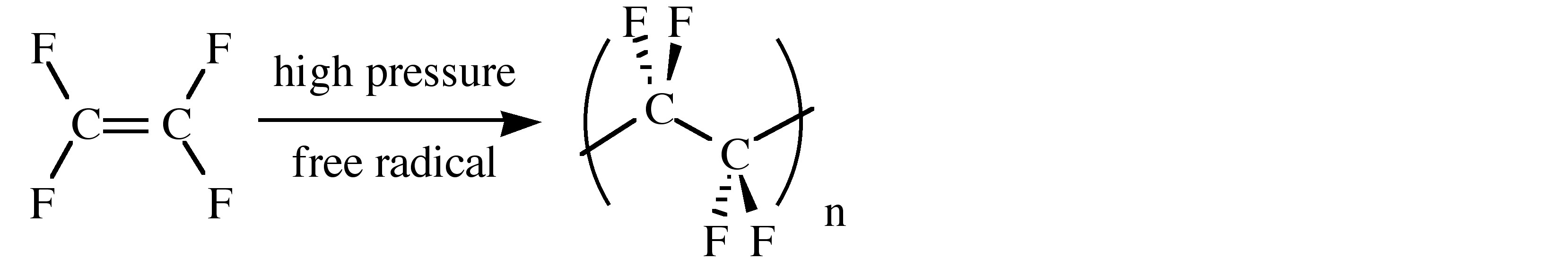
The physical properties of fluorocarbons range from inert to toxic. Thus, poly(tetrafluoroethylene), (C2F4)n, known by either its acronym (PTFE) or its trade name (Teflon), is chemically inert and has a low coefficient of friction (Table \(\PageIndex{3}\)). As a consequence its uses include coatings on armor-piercing bullets (to stop the wear on the gun barrel), laboratory containers and magnetic stirrers, tubing for corrosive chemicals, and thread seal tape in plumbing applications (plumbers tape). A summary of the physical properties of PTFE is given in Table \(\PageIndex{3}\). PTFE is synthesized by the emulsion polymerization of tetrafluoroethylene monomer under pressure through free radical catalyst.
| Property | Value |
| Density | 2.2 g/cm3 |
| Melting point | 327 °C |
| Young's modulus | 0.5 GPa |
| Yield strength | 23 MPa |
| Coefficient of friction | 0.05 - 0.10 |
| Dielectric constant | 2.1 |
| Dielectric strength (1 MHz) | 60 MV/m |
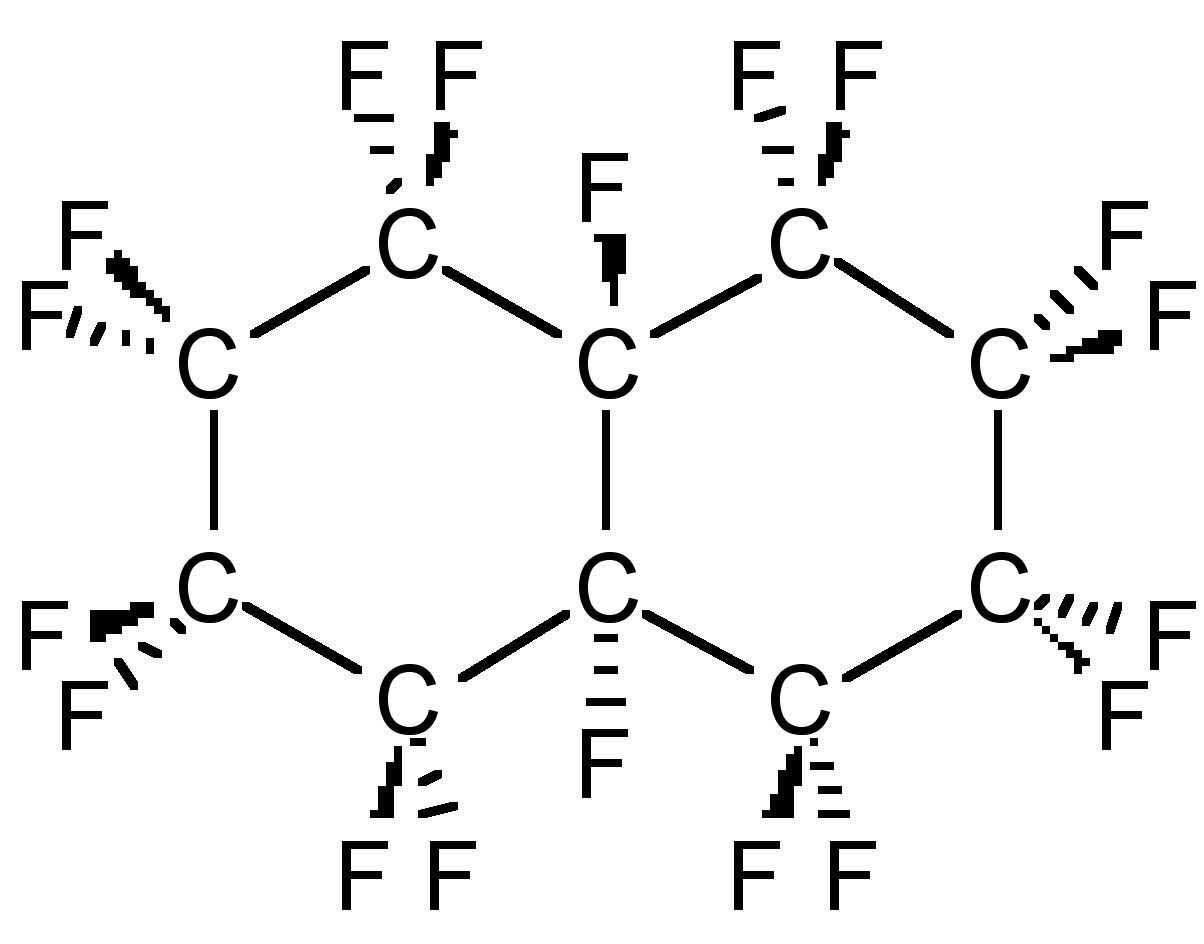
In contrast with PTFE, octafluoroisobutylene, (CF3)2C=CF2, is highly toxic, while perfluorodecahydronapthalene (C10F8, Figure \(\PageIndex{1}\)) is used as a blood substitute component.
Mixed halides
Mixed halides are an important class of halocarbon compound. They are synthesized by halide exchange, (7.8.6). The high cost of SbF3 means that the reaction is generally run with an excess of the chloride.
\[\text{3 CCl}_4 \text{ + 2 SbF}_3 \rightarrow \text{2 CCl}_2\text{F}_2 \text{ + 2 SbCl}_2\]
The ordinary name for mixed carbon halide is halon or Freon, although Freon is actually a Du Pont trademark. A list of selected Freon compounds are given in Table \(\PageIndex{4}\). Halons are non-toxic, non-flammable, and have no odor. However, it is their very lack of reactivity that has caused a problem.
| Freon | Formula | Uses |
| 12 | CCl2F2 | Refrigerant |
| 11 | CCl3F | Refrigerant |
| 114 | ClF2C-CClF2 | Refrigerant |
| 113 | Cl3C-CF3 | Solvent |
| 13B1 | CBrF3 | Fire extinguisher |
| 1211 | CBrClF2 | Fire extinguisher |
Environmental impact of chlorofluorcarbon compounds (CFCs)
Chlorofluorcarbon compounds (CFCs) are very stable and are not degraded in the environment. As a consequence they are transported to the stratosphere where they decomposed upon photolysis, (7.8.7). The resulting chloride radical is a catalyst for the decomposition of ozone, (7.8.8), as well as a catalyst for the reaction of ozone with molecular oxygen, (7.8.9).
\[ \text{CCl}_2\text{F}_2\text{ + h}\nu \rightarrow \text{CClF}_2\text{ + Cl}\cdot \]
\[ \text{2 O}_3 \xrightarrow{\text{Cl}\cdot} \text{3 O}_2\]
\[ \text{O}_3 \text{ + O} \xrightarrow{\text{Cl}\cdot} \text{2 O}_2 \]
The widespread use of CFCs as refrigerants and propellants meant that by 1986 there were 2.5 billion pounds of CFC being liberated to the atmosphere. This was equivalent to 1/2. lb per person on the planet. Since the ozone layer provides the vital protection to life on the Earth’s surface from high energy UV radiation the release of CFC (along with other chemicals) caused a dramatic change in the ozone layer, including the increase in the polar hole in the ozone layer. As a result of the EU called for a complete ban of CFCs (which was followed by other countries). In their place new chemicals with similar refrigerant properties were developed. These compounds contained C-H bonds (e.g., C2HCl2F3 and C2H3Cl2F) that are readily broken in the lower atmosphere, thus limiting the transport to the stratosphere.
Carbonyl halides
All the carbonyl halides (X2C=O, X = F, Cl, Br, I) are known (Table \(\PageIndex{1}\)). Phosgene (Cl2C=O) was first synthesized by John Davy (Figure \(\PageIndex{2}\)) in 1812 by exposing a mixture of carbon monoxide and chlorine to sunlight, (7.8.10). He named it phosgene from the Greek, phos (light) and gene (born), in reference to use of light to promote the reaction. The fluoride is also prepared by the reaction of carbon monoxide with the halogen, while the bromide is prepared by the partial hydrolysis of CBr4 with sulfuric acid.
\[ \text{CO + Cl}_2 \rightarrow \text{Cl}_2\text{C=O} \]

The synthesis of isocyanates from alkyl or aryl amines illustrates the electrophilic character of phosgene and its ability to introduce the equivalent of "CO2+", (7.8.11). This reaction is conducted in the presence of a base such as pyridine that absorbs the hydrogen chloride. Phosgene may also be used to produce acyl chlorides from carboxylic acids, (7.8.12). However, thionyl chloride is more commonly and more safely used in this reaction.
\[ \text{RNH}_2 \text{ + Cl}_2\text{C=O} \rightarrow \text{RN=C=O + 2 HCl} \]
\[ \text{RCO}_2\text{H + Cl}_2\text{C=O} \rightarrow \text{RC(O)Cl + HCl + CO}_2 \]
Phosgene as a weapon of war
Phosgene is a toxic gas with the smell of “new-mown hay” and was used in chemical warfare during the First World War (Figure \(\PageIndex{3}\)) where it was a more potent weapon than chlorine. While chlorine was potentially deadly it caused the victim to violently cough and choke (the bodies natural defense to limiting inhalation), in contrast, phosgene caused much less coughing with the result that more of it was inhaled. Phosgene often had a delayed effect; apparently healthy soldiers were taken down with phosgene gas poisoning up to 48 hours after inhalation. A fatal dose of phosgene eventually led to shallow breathing and retching, pulse up to 120, an ashen face and the discharge of four pints of yellow liquid from the lungs each hour for the 48 of the drowning spasms.

Although phosgene’s boiling point (7.6 °C) meant that is was a vapor, the so-called "white star" mixture of phosgene and chlorine was commonly used on the Somme, because the chlorine supplied the necessary vapor with which to carry the phosgene. A summary of the casualties inflicted by chemical warfare agents during the Great War is shown in Table \(\PageIndex{5}\).
| Country | Total casualties | Deaths |
| Russia | 419,340 | 56,000 |
| Germany | 200,000 | 9,000 |
| France | 190,000 | 8,000 |
| British Empire | 188,706 | 8,109 |
| Austria-Hungary | 100,000 | 3,000 |
| USA | 72,807 | 1,462 |
| Italy | 60,000 | 4,627 |
| Others | 10,000 | 1,000 |

