7.6: Carbon Dioxide
- Page ID
- 212656
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Carbon dioxide (CO2) is the most stable oxide of carbon and is formed from the burning of carbon or carbon containing compounds in air or an excess of oxygen, (7.6.1). For industrial applications it is usually prepared from the decomposition of calcium carbonate (limestone), (7.6.2), rather than separation from combustion products.
\[ \text{C + O}_2 \rightarrow \text{CO}_2\]
\[ \text{CaCO}_3 \rightarrow \text{CaO + CO}_2\]
Phase chemistry of carbon dioxide
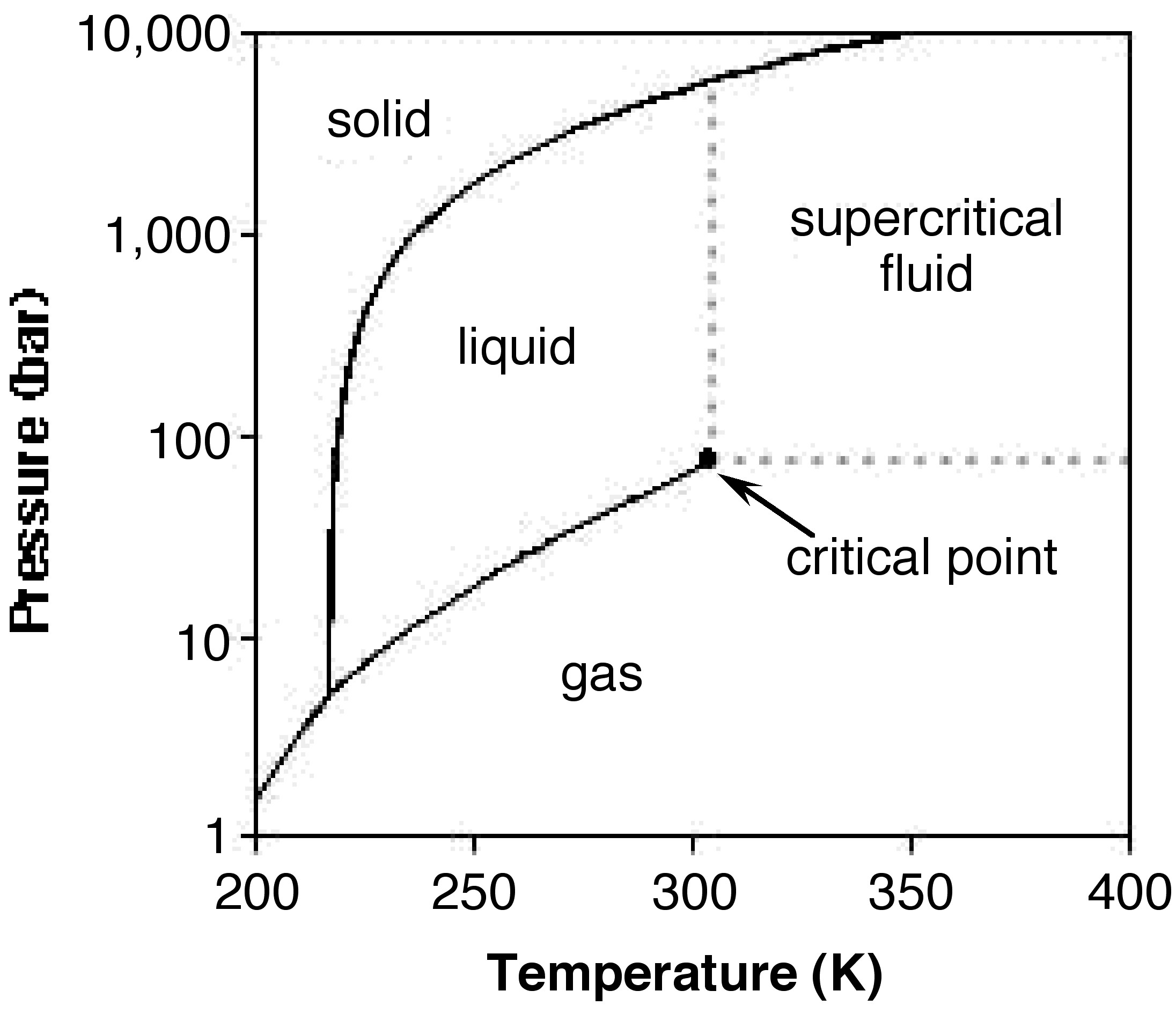
Carbon dioxide does not exists as a liquid under normal atmospheric pressure, but solid CO2 (also known as dry ice) sublimes at -78.5 °C (Figure \(\PageIndex{1}\)). Dry ice (Figure \(\PageIndex{2}\)) is commonly used as a refrigerant for food or biological sample preservation.

Note
When dry ice is placed in water (especially heated) sublimation is accelerated, and a low-sinking dense cloud of fog (smoke-like) is created. This is used in fog machines, at theaters, concerts, haunted houses, and nightclubs for dramatic effects (Figure \(\PageIndex{3}\)). Fog from dry ice hovers above the ground unlike other artificial fog machines (that use partial combustion of oil) where the fog rises like smoke.

Supercritical carbon dioxide
As noted above carbon dioxide usually behaves as a gas in air at standard temperature and pressure (STP = 25 °C and 1 atm) or as a solid when frozen. However, if the temperature and pressure are both increased from STP to be at or above the critical point (Figure \(\PageIndex{1}\)), carbon dioxide adopts properties midway between a gas and a liquid (Tc = 31.1 °C and Pc = 72.9 atm).
Supercritical CO2 has become an important industrial solvent due to its role in chemical extraction in addition to its low toxicity and environmental impact. In this regard it is seen as a promising green solvent. One of the biggest applications is the decaffeination of coffee and tea without leaving any residue and allowing the caffeine to be separated and used in other beverage products.
Structure and bonding
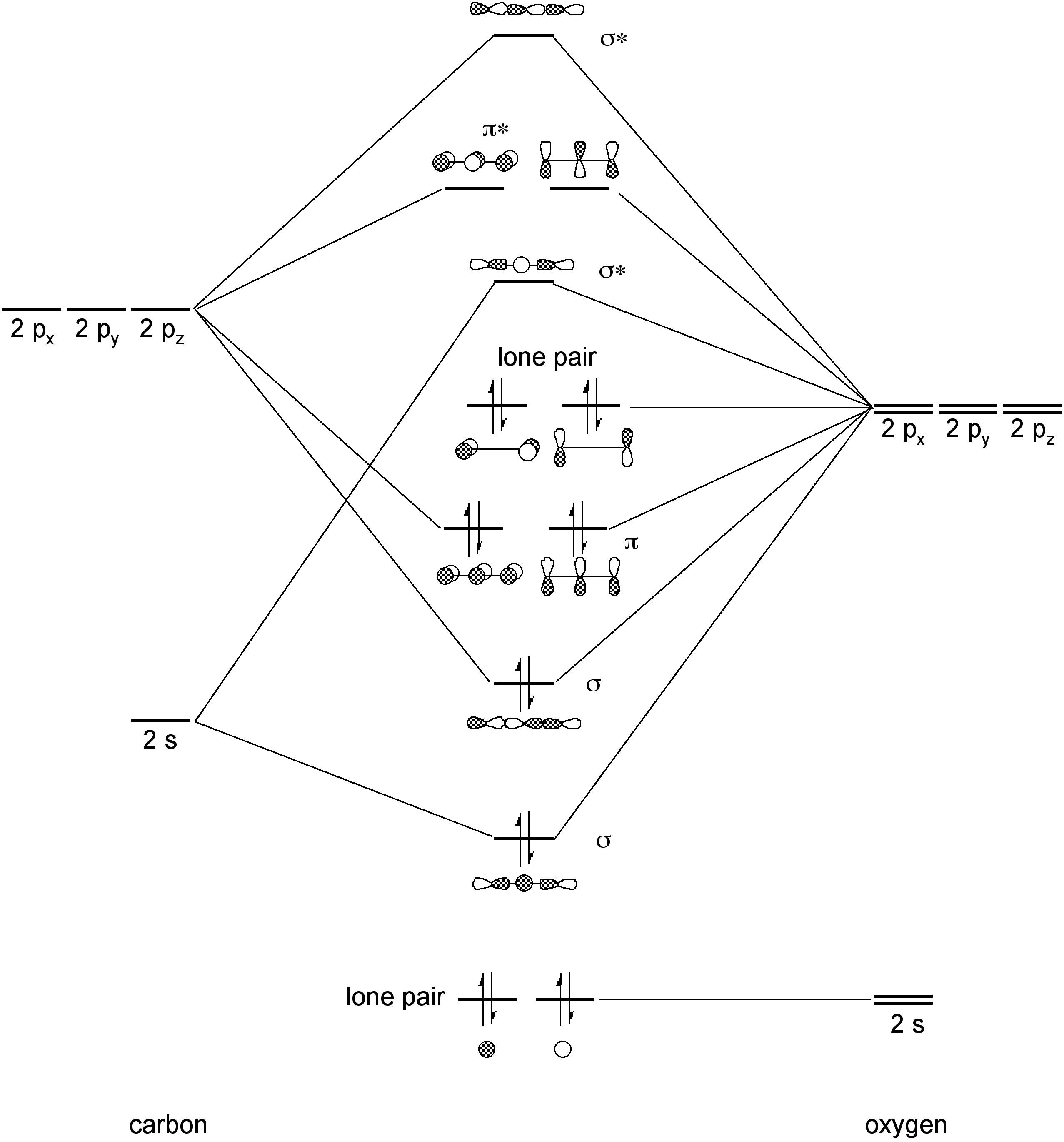
Carbon dioxide is a linear molecule due to π-localization. The bonding in CO2 involves 2 σ-bond and 2 sets of 3 center π-bonds (Figure \(\PageIndex{4}\)). The C-O bond length of 1.2 Å should be compared to the value observed for organic carbonyls (e.g., ketones, esters, aldehydes) of 1.2 – 1.3 Å.
Dissolution and reaction with water
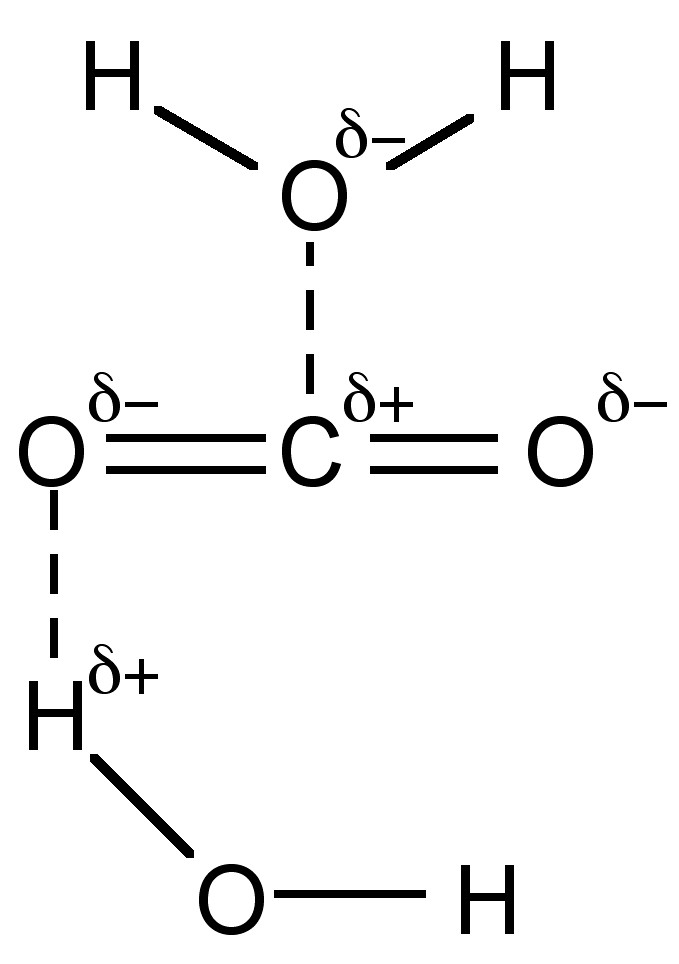
Although CO2 has no dipole moment it is very polar (dielectric constant = 1.60 at 0 °C, 50 atm) and consequently dissolves in polar solvents such as water up to a concentration of 18% (0.04 M). Most of it (+99%) is present as solvated CO2 (Figure \(\PageIndex{5}\)), and only ca. 0.2% is reacted to form carbonic acid, (7.6.1), with subsequent equilibria resulting in the formation of bicarbonate (HCO3-) and carbonate (CO32-).
\[ \text{H}_2\text{O + CO}_2 \rightarrow \text{H}_2\text{CO}_3\]
The overall reaction involves a series of equilibria. The first equilibrium is the formation of carbonic acid, (7.6.4). The reaction rates, (7.6.5), are on the magnitude of 1 second (i.e., slow), and as a consequence when carbon dioxide is carried in the body an enzyme is present to speed up the reaction.
\[ \text{CO}_{\text{2(solv)}} \text{ + H}_2\text{O} \xrightleftharpoons[\text{k}_{\text{H}_2\text{CO}_3}]{\text{k}_{\text{CO}_2}} \text{H}_2\text{CO}_3\]
\[ \text{K = } \dfrac{\text{k}_{\text{H}_2\text{CO}_3}}{\text{k}_{\text{CO}_2}} \text{ = } \dfrac{25}{0.04} \text{ = 600}\]
The 2nd equilibrium is as a consequence of first ionization of carbonic acid to form bicarbonate (HCO3-), (7.6.6). In contrast to the first reaction, (7.6.4), this reaction is very fast with a Keq = 1.6 x 10-4 @ 25 °C.
\[ \text{H}_2\text{CO}_3 \rightleftharpoons \text{HCO}_3^- \text{ + H}^+ \]
The 3rd equilibrium involves the formation of the carbonate ion (7.6.7), and has a Keq = 4.84 x 10-11. Carbonate (CO32-) is a delocalized ligand, which can act as a mono or bidentate or bridging group.
\[ \text{HCO}_3^- \rightleftharpoons \text{CO}_3^{2-} \text{ + H}^+ \]
The formation of carbonic acid is the reason that even in the absence of pollutants (such as SO2) natural rain water is slightly acidic due to dissolved CO2. The equilibrium associated with carbonic acid is also responsible for the buffering of the pH in blood.
Reaction chemistry
Photosynthesis in plants reduces CO2 to organic matter but similar reactions have yet to be developed in non-living systems.
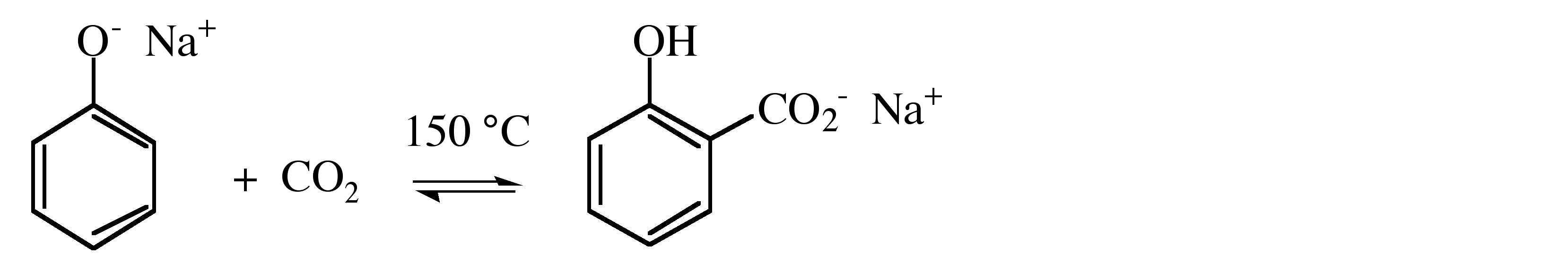
Grignards react readily with carbon dioxide to form the carboxylate, which yields the associated carboxylic acid upon hydrolysis, (7.6.8). Similar reactions occur with other organometallic compounds. In addition, CO2 reacts with alkali metal salts of phenols (phenolates) to yield the hydroxy-carboxylate.
\[ \text{RMgX + CO}_2 \rightarrow \text{RCO}_2\text{MgX} \xrightarrow{\text{H}_2\text{O}} \text{RCO}_2\text{H + HOMgX}\]
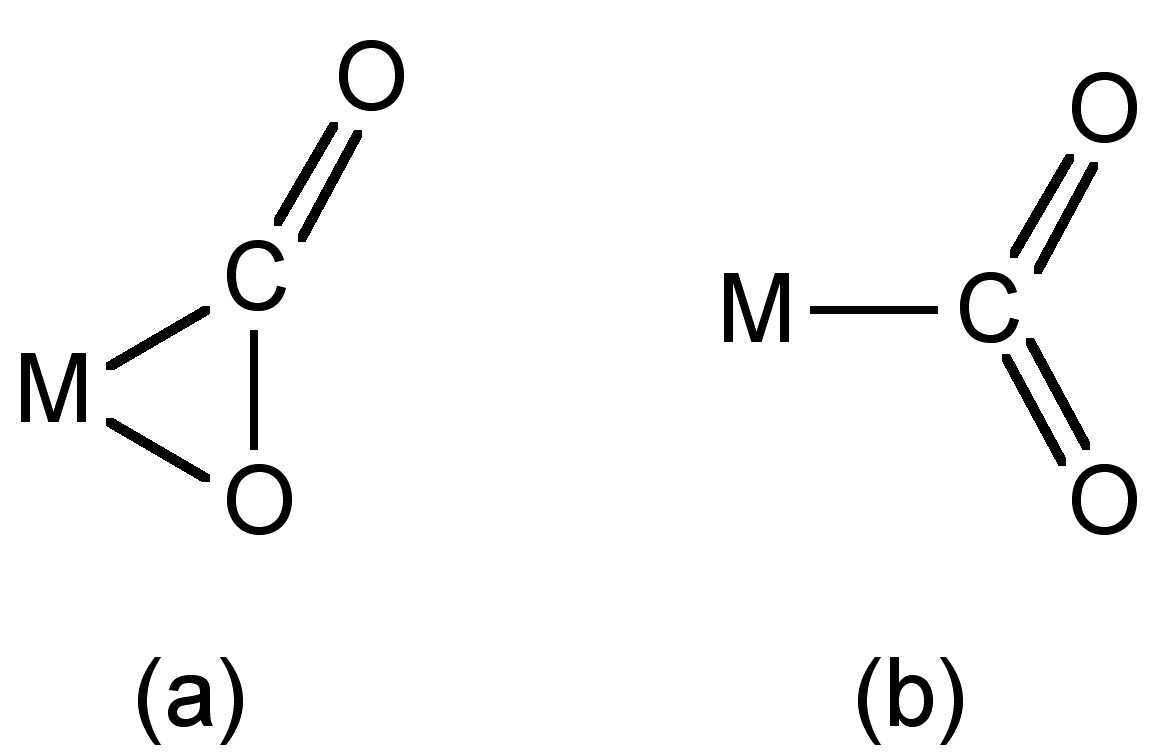
A number of complexes of CO2 with transition metals are known in which the coordination can occur via the central carbon (Figure \(\PageIndex{6}\)a) or the C=O bond (Figure \(\PageIndex{6}\)b). Alternatively, CO2 can bridge two metal centers.
Global warming and carbon dioxide
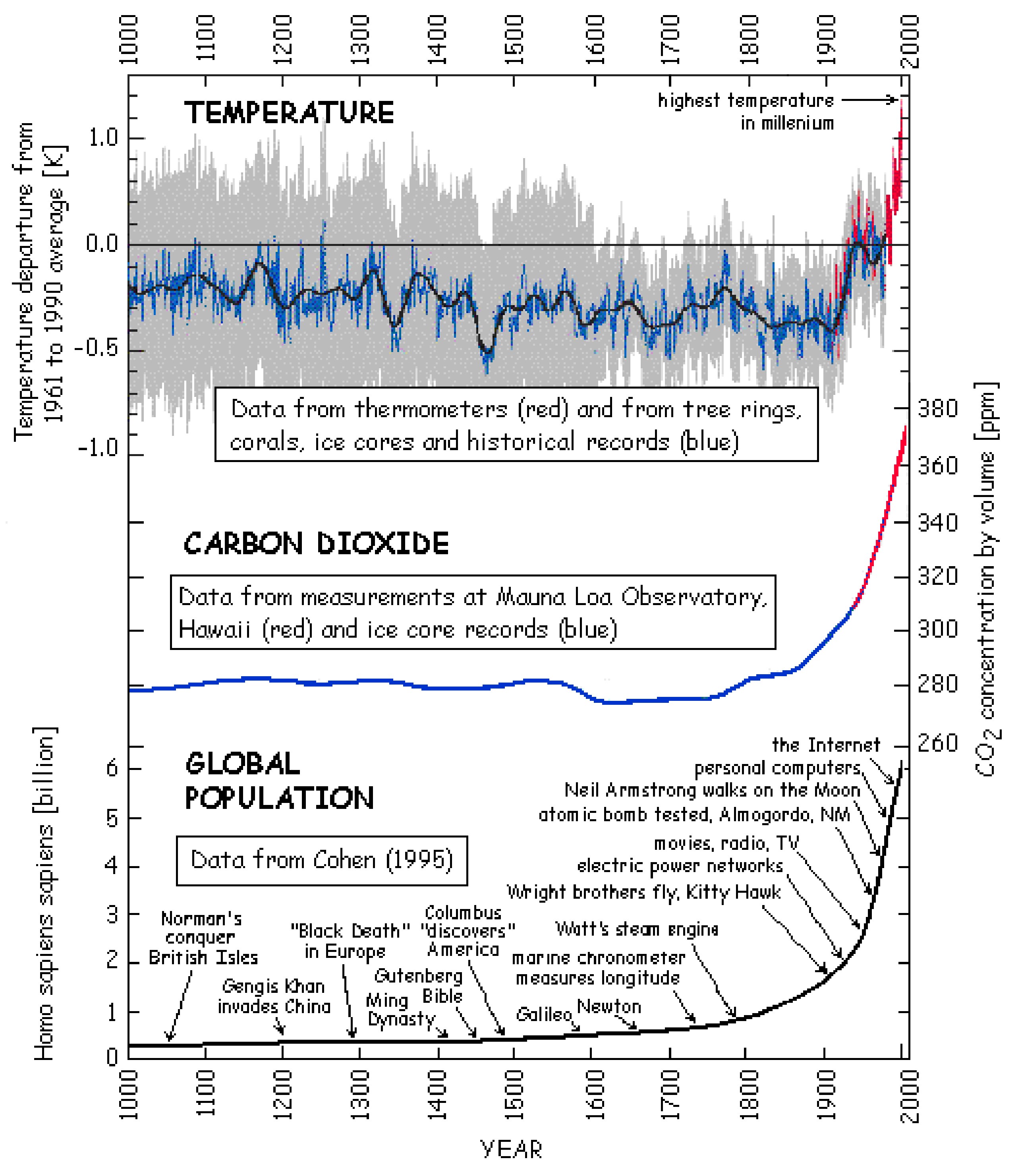
Global warming is the process of the observed increase in the average temperature of the Earth's near-surface air and oceans since the mid-20th century. Global surface temperature increased 0.74 °C (1.33 °F) between the start and the end of the 20th century (Figure \(\PageIndex{7}\)). It is generally agreed that the majority of this temperature increase has occurred since the middle of the 20th century and was caused by increasing concentrations of greenhouse gases resulting from burning fossil fuels (the generation of additional CO2) and deforestation (the loss of a mechanism for the consumption of CO2), see Figure \(\PageIndex{7}\). While it is appreciated that natural phenomena (including solar radiation and volcanoes) produced most of the warming from pre-industrial times, the magnitude of the changes brought on by global industrialization is more significant.
The Earth’s atmosphere has two functions. First, the ozone (O3) in the upper atmosphere screens harmful UV from reaching the surface of the Earth. Second, as solar radiation penetrates the atmosphere a portion of the heat is then retained as a consequence of the CO2 in the atmosphere. It is this process that modulates the surface temperature and provides a stable environment for life. The failure or alteration of either of these processes can have a dramatic effect on the livability of a planet.
Consider the relative position of Venus, Earth, and Mars to the Sun (Figure \(\PageIndex{8}\)). The closer a planet is to the sun the greater the UV radiation and the greater the heating of the planet; however, the temperature is also greatly modulated by the atmosphere. Venus has an atmosphere comprising 95% CO2 and has a surface temperature of approximately 450 °C. In contrast, while Mars’ atmosphere is also 95% CO2, it is only 1% as dense as that of Earth’s, and thus the surface temperatures range from 40 °C during the day (due to radiative heating) to –80 °C at night (due to the lack of retained heat because of the thin atmosphere). These should be compared to Earth’s atmosphere which is 0.038% CO2, which allows for the correct amount of heat to be retained to sustain life. Clearly any significant change in the CO2 content of the atmosphere will change the global temperatures of a planet.
Bibliography
- N. Stern, The Stern Review: The Economics of Climate Change, HM Treasury, London.
- R. B. Gupta and J.-J. Shim, Solubility in Supercritical Carbon Dioxide, CRC Press (2006).
- Carbon Dioxide Capture and Storage: Special Report of the Intergovernmental Panel on Climate Change, Cambridge University Press (2005).


