1.10: Periodic Trends for the Main Group Elements
- Page ID
- 214086
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Within the main group (s- and p-block) elements of the Periodic Table (Figure \(\PageIndex{1}\).66) there are some general trends that we can observe for the elemental form, as well as the hydrides, oxides, and halides.
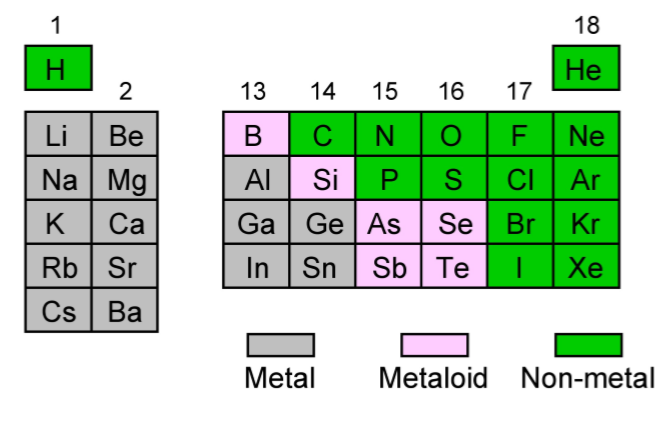
Periodic trends for the main group elements
Within the main group (s- and p-block) elements there are some general trends that we can observe.
- The further down a given Group the elements have increased metallic character, i.e., good conductors of both heat and electricity, and exhibit delocalized bonding.
- Moving from left to right across a Period the elements have greater non-metallic character, they are insulators with localized bonding.
- Within the p-block at the boundary between the metallic elements (Figure \(\PageIndex{1}\).66, grey elements) and nonmetal elements (Figure \(\PageIndex{1}\).66, green elements) there is positioned boron and silicon that are metalloid in character (Figure \(\PageIndex{1}\).66, pink elements), i.e., they have low electrical conductivity but it increases with temperature.
As an example of these changes Table \(\PageIndex{1}\).12 shows the trends across one Period.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| Properties | Electropositive metal | Electropositive metal | Metal but forms covalent bonds | Metalloid semiconductor metal/non-metal characteristics | E-E bonding in elements | E-E bonding in elements | Simple molecule | Monoatomic gas |
Periodic trends for the main group hydrides
The properties of main group hydrides are dependant on the difference in electronegativity between the element and hydrogen (Table \(\PageIndex{1}\).13). Elements on the left of the Periodic Table are highly electropositive and form ionic hydrides, while those of the center and right are covalent in character. However, of those with covalent E-H bonds, there is a change from polymeric hydrides to molecular compounds. For example, the Group 13 element hydrides (i.e., BH3) form hydrogen-bridged oligomers (i.e., B2H6). In contrast, HCl is a diatomic molecule.
| Hydride | Element electronegativity | Hydrogen electronegativity | E-H polarity | Structure | Comments |
| NaH | 0.9 | 2.1 | M+H- | Ionic | Reacts with H2O to liberate H2 |
| BH3 | 2.0 | 2.1 | Bδ+-Hδ- | Oligomeric and polymeric | Reacts slowly with H2O |
| CH4 | 2.4 | 2.1 | Cδ--Hδ+ | Molecular | Insoluble in H2O |
| HCl | 3.0 | 2.1 | Clδ--Hδ+ | Molecular | Dissolves in H2O to form H+ and Cl- |
Periodic trends for the main group oxides
As with hydrides the properties of main group oxides are dependant on the difference in electronegativity between the element and oxygen. Highly electropositive metals for ionic oxides, while other elements for covalent bonds (albeit polar in character) with oxygen. In addition, the aggregation of covalent oxides decreased across the Period from left to right (Table \(\PageIndex{1}\).14). As may also be seen from Table \(\PageIndex{1}\).14, oxides of elements on the left of the Periodic Table dissolve in water to form basic solutions, while those on the right form acidic solutions. There is a class of oxides (especially those of Group 13 and 14) that can react as either an acid or a base. These are known as amphoteric substances.
Note
The word is from the Greek prefix ampho meaning "both"
| Oxide | Bonding | Reactivity with H2O |
Description |
| Na2O | Ionic | Dissolves to give a strong base | Basic |
| Al2O3 | Covalent polymeric | Dissolves in both acidic and basic solution | Amphoteric |
| SiO2 | Covalent polymeric | Dissolves in both acidic and basic solution | Amphoteric |
| CO2 | Covalent molecular | Dissolves to give a weak acid | Acidic |
| SO3 | Covalent molecular | Dissolves to give a strong acid | Aci |
In summary, oxides of the main group elements show two trends.
- From left to right across a Period, the oxides change from ionic → oligomeric/polymeric covalent → molecular covalent
- From left to right across a Period, the oxides change from ionic → oligomeric/polymeric covalent → molecular covalent.
Periodic trends for the main group chlorides
The trend between ionic and non-ionic/covalent in moving across a Period is also true for the chlorides of the main group elements. Those on the left (i.e., Group 1 and 2) are ionic and soluble in water, while those to the right tend to give acidic solutions due to reactions with the water and the formation of hydrochloric acid, e.g., (1.10.1).
\[SiCl_4 + 2 H_2O \rightarrow SiO_2 + 4 HCl_{(aq)}\]


