1.1: Lattices
- Page ID
- 474754
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In two-dimensional (2-d) real space, a lattice can be described in one of three ways:
- an infinite, discrete set of points {[n1, n2]};
- an infinite, discrete set of vectors \(\left\{{\boldsymbol T}_{n_{1}n_{2}} \right\}\); and
- an infinite set of space-filling (non-overlapping) identical unit cells.

For 2-d space, there are two linearly independent basis vectors \(\textbf{a}_1\) and \(\textbf{a}_2\) between adjacent lattice points, so that every lattice vector \({\boldsymbol T}_{n_{1}n_{2}} = n_{1}{\boldsymbol a}_{1} + n_{2}{\boldsymbol a}_{2}\) for any pair of integers \(\textbf{n}_1\) and \(\textbf{n}_2\). The basis vectors form a parallelogram with sides \(\textbf{a}_1\) and \(\textbf{a}_2\) and interior angle α3. Although selection of the origin point [0, 0] is arbitrary, it is chosen to be a site of either highest point symmetry or inversion of the overall crystalline structure. Often, these two characteristics coincide, but sometimes these two positions differ. In the illustration, the lattice points \([\textbf{n}_1, \textbf{n}2]\) are at the corners of the unit cells and are shared by 4 adjacent unit cells. As a result of the space-filling nature of these shapes, each lattice point belongs to one unit cell, the so-called primitive cell. In other words, the area of one primitive unit cell contains one and only one lattice point of the crystalline lattice. As the figure indicates, basis vectors are not unique because there are different shapes that have the same area. However, the standard choice of unit cell is to use the smallest independent distances and an angle falling between 90° and 120° inclusive.
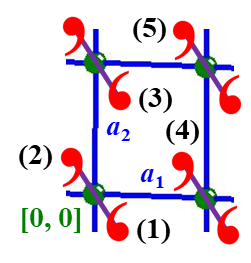
Once the unit cell (basis) vectors are chosen, then every point r in 2-d space can be identified by fractional coordinates [x, y] relative to the origin [0, 0]: r = xa1 + ya2. Points within the unit cell assigned to the origin have fractional coordinates 0 ≤ x < 1 and 0 ≤ y < 1. Therefore, \({\boldsymbol r} + {\boldsymbol T}_{n_{1}n_{2}} = \left( x + n_{1} \right){\boldsymbol a}_{1} + \left( y + n_{2} \right){\boldsymbol a}_{2}\). The figure below illustrates the application of fractional coordinates to locate five different points of the structure with respect to the origin point:
- A visual estimate is [+0.20, –0.25]: 0.20a1 – 0.25a2.
- Related to (1) via inversion [–0.20, +0.25]: –0.20a1 + 0.25a2.
- Related to (1) by translation along a2 [+0.20, +0.75]: 0.20a1 + 0.25a2.
- Related to (2) by translation along a1 [+0.80, +0.25]: 0.80a1 + 0.25a2.
- Related to (2) by translation a1 + a2 [+0.80, +1.25]: 0.80a1 + 1.25a2.
2-d Lattices are useful for identifying translational periodicity of surfaces, 2-d structures like graphene, and 2-d projections of 3-d crystals.
In three-dimensional (3-d) real space, a lattice can also be described in one of three ways:
- an infinite, discrete set of points {[n1, n2, n3]};
- an infinite, discrete set of vectors \(\left\{{\boldsymbol T}_{n_{1}n_{2}n_{3}} \right\}\); and
- an infinite set of space-filling (non-overlapping) identical unit cells.

Now, there are three linearly independent basis vectors a1, a2, and a3 between adjacent lattice points, so that every lattice vector \({\boldsymbol T}_{n_{1}n_{2}n_{3}} = n_{1}{\boldsymbol a}_{1} + n_{2}{\boldsymbol a}_{2} + n_{3}{\boldsymbol a}_{3}\) for any triplet of integers n1, n2, and n3. The basis vectors form a parallelepiped with sides a1, a2, and a3 and interior angles α1 (between a2 and a3), α2 (between a1 and a3), and α3 (between a1 and a2). This region is a primitive unit cell because it belongs to one lattice point and its origin, albeit arbitrary, falls at the site of either highest point symmetry or inversion. In the figure, the lattice points are at the 8 unit cell corners, which are each shared with 8 adjacent cells. Therefore, the volume of one primitive unit cell contains one and only one lattice point. Like 2-d lattices, the basis vectors of 3-d lattices are not unique, but the standard choice is to use the smallest independent distances and angles ranging between 90° and 120° inclusive. Conventional crystallographic descriptions of unit cell vectors use the symbols a, b, and c: lengths a, b, c, and angles α, β, γ.


