26.6: Aromatic Hydrocarbons
- Page ID
- 24384
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Next we consider a class of hydrocarbons with molecular formulas like those of unsaturated hydrocarbons, but which, unlike the alkenes, do not readily undergo addition reactions. These compounds comprise a distinct class, called aromatic hydrocarbons, with unique structures and properties. We start with the simplest of these compounds. Benzene (C6H6) is of great commercial importance. The formula C6H6 seems to indicate that benzene has a high degree of unsaturation. (Hexane, the saturated hydrocarbon with six carbon atoms has the formula C6H14—eight more hydrogen atoms than benzene.) However, despite the seeming low level of saturation, benzene is rather unreactive. It does not, for example, react readily with bromine, which, is a test for unsaturation.
Benzene is a liquid that smells like gasoline, boils at 80°C, and freezes at 5.5°C. It is the aromatic hydrocarbon produced in the largest volume. It was formerly used to decaffeinate coffee and was a significant component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. It was removed from many product formulations in the 1950s, but others continued to use benzene in products until the 1970s when it was associated with leukemia deaths. Benzene is still important in industry as a precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent for such things as cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is a natural constituent of petroleum products, but because it is a known carcinogen, its use as an additive in gasoline is now limited.
To explain the surprising properties of benzene, chemists suppose the molecule has a cyclic, hexagonal, planar structure of six carbon atoms with one hydrogen atom bonded to each. We can write a structure with alternate single and double bonds, either as a full structural formula or as a line-angle formula:
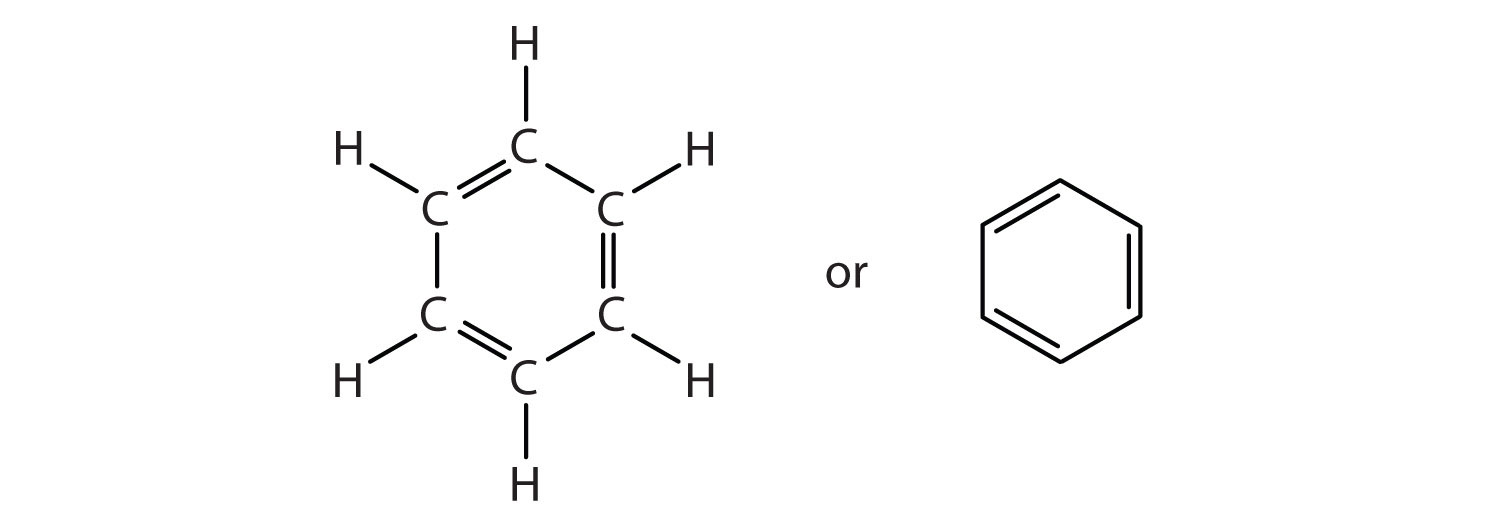
However, these structures do not explain the unique properties of benzene. Furthermore, experimental evidence indicates that all the carbon-to-carbon bonds in benzene are equivalent, and the molecule is unusually stable.
Chemists often represent benzene as a hexagon with an inscribed circle:
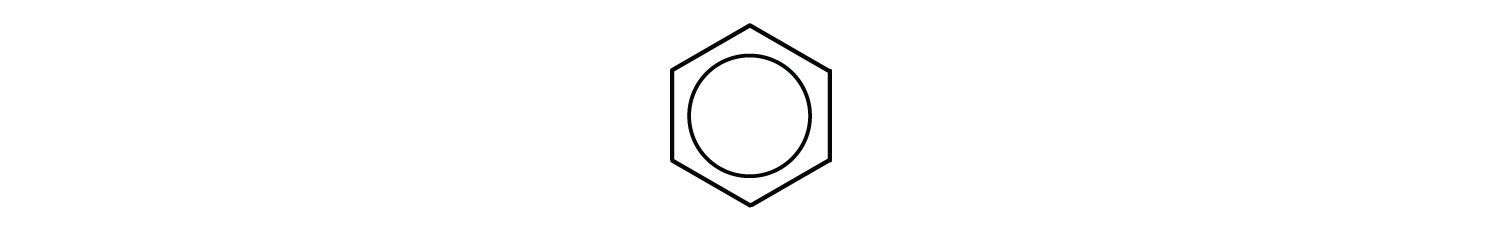
The inner circle indicates that the valence electrons are shared equally by all six carbon atoms (that is, the electrons are delocalized, or spread out, over all the carbon atoms). It is understood that each corner of the hexagon is occupied by one carbon atom, and each carbon atom has one hydrogen atom attached to it. Any other atom or groups of atoms substituted for a hydrogen atom must be shown bonded to a particular corner of the hexagon. We use this modern symbolism, but many scientists still use the earlier structure with alternate double and single bonds.
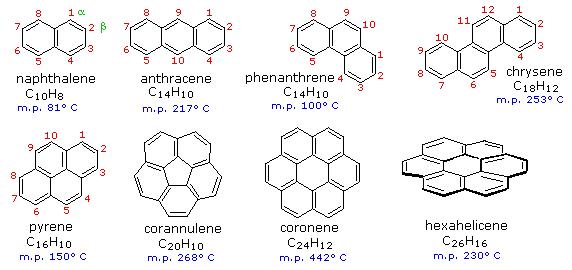
Benzene rings may be joined together (fused) to give larger polycyclic aromatic compounds. A few examples are drawn below, together with the approved numbering scheme for substituted derivatives. The peripheral carbon atoms (numbered in all but the last three examples) are all bonded to hydrogen atoms. Unlike benzene, all the C-C bond lengths in these fused ring aromatics are not the same, and there is some localization of the pi-electrons.
The six benzene rings in coronene are fused in a planar ring; whereas the six rings in hexahelicene are not joined in a larger ring, but assume a helical turn, due to the crowding together of the terminal ring atoms. This helical configuration renders the hexahelicene molecule chiral, and it has been resolved into stable enantiomers.
Aromaticity
Aromaticity is a property of conjugated cycloalkenes in which the stabilization of the molecule is enhanced due to the ability of the electrons in the orbitals to delocalize. This act as a framework to create a planar molecule. Why do we care if a compound is aromatic or not? Because we encounter aromatics every single day of our lives. Without aromatic compounds, we would not only be lacking many material necessities, our bodies would also not be able to function. Aromatic compounds are essential in industry; about 35 million tons of aromatic compounds are produced in the world every year to produce important chemicals and polymers, such as polyester and nylon. Aromatic compounds are also vital to the biochemistry of all living things. Three of the twenty amino acids used to form proteins ("the building blocks of life") are aromatic compounds and all five of the nucleotides that make up DNA and RNA sequences are all aromatic compounds. Needless to say, aromatic compounds are vital to us in many aspects.
The three general requirements for a compound to be aromatic are:
- The compound must be cyclic
- Each element within the ring must have a p-orbital that is perpendicular to the ring, hence the molecule is planar.
- The compound must follow Hückel's Rule (the ring has to contain 4n+2 p-orbital electrons).
Among the many distinctive features of benzene, its aromaticity is the major contributor to why it is so unreactive. This section will try to clarify the theory of aromaticity and why aromaticity gives unique qualities that make these conjugated alkenes inert to compounds such as Br2 and even hydrochloric acid. It will also go into detail about the unusually large resonance energy due to the six conjugated carbons of benzene.
The delocalization of the p-orbital carbons on the sp2 hybridized carbons is what gives the aromatic qualities of benzene.
.bmp?revision=1)
Because of the aromaticity of benzene, the resulting molecule is planar in shape with each C-C bond being 1.39 Å in length and each bond angle being 120°. You might ask yourselves how it's possible to have all of the bonds to be the same length if the ring is conjugated with both single (1.47 Å) and double (1.34 Å), but it is important to note that there are no distinct single or double bonds within the benzene. Rather, the delocalization of the ring makes each count as one and a half bonds between the carbons which makes sense because experimentally we find that the actual bond length is somewhere in between a single and double bond. Finally, there are a total of six p-orbital electrons that form the stabilizing electron clouds above and below the aromatic ring.
Aromatic Hydrocarbon Nomenclature
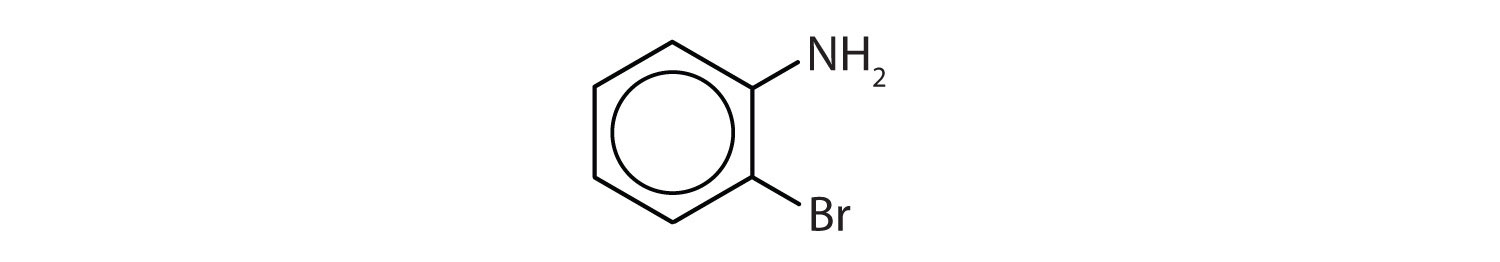
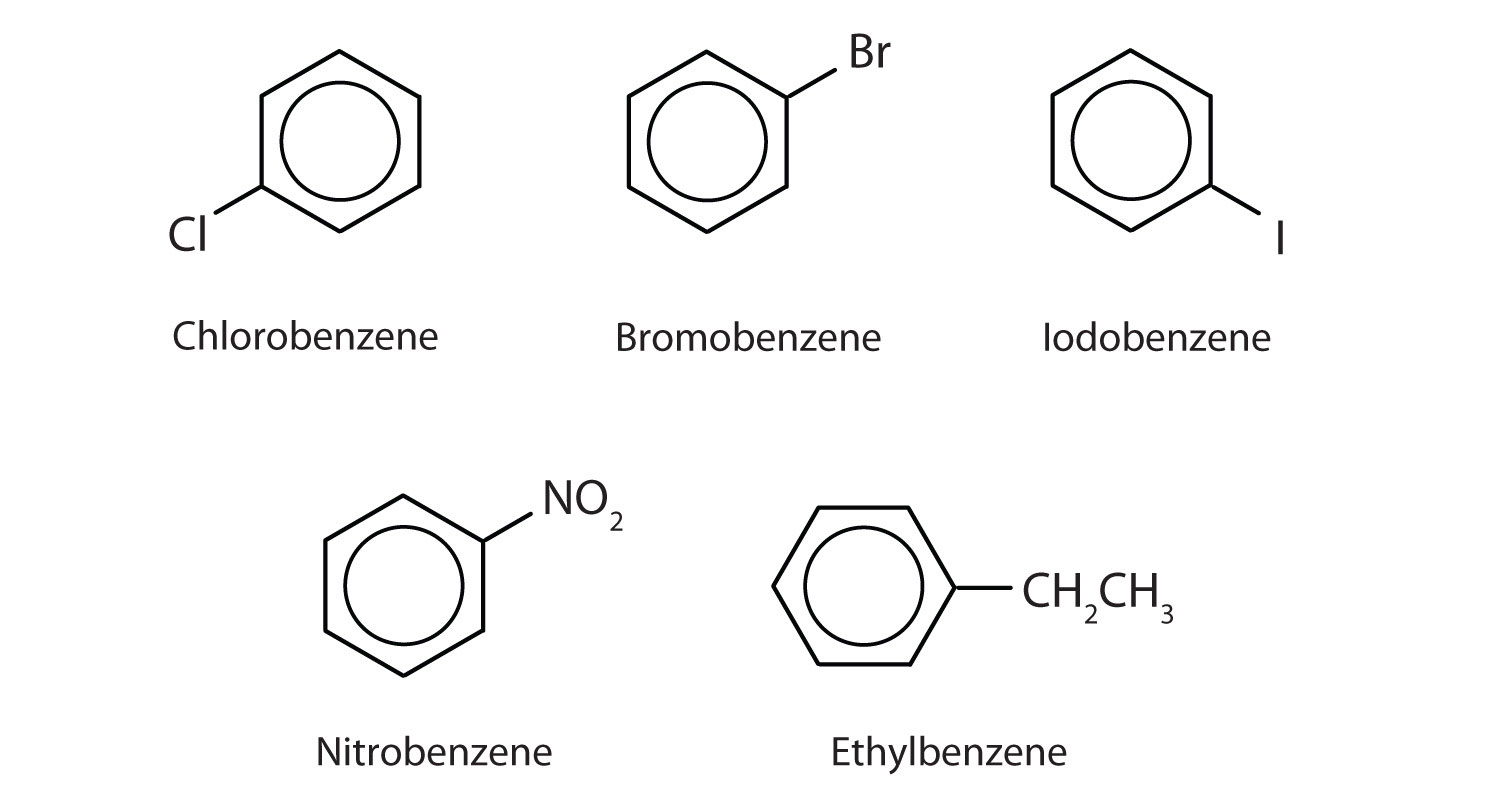
In the International Union of Pure and Applied Chemistry (IUPAC) system, aromatic hydrocarbons are named as derivatives of benzene. Figure \(\PageIndex{1}\) shows four examples. In these structures, it is immaterial whether the single substituent is written at the top, side, or bottom of the ring: a hexagon is symmetrical, and therefore all positions are equivalent.
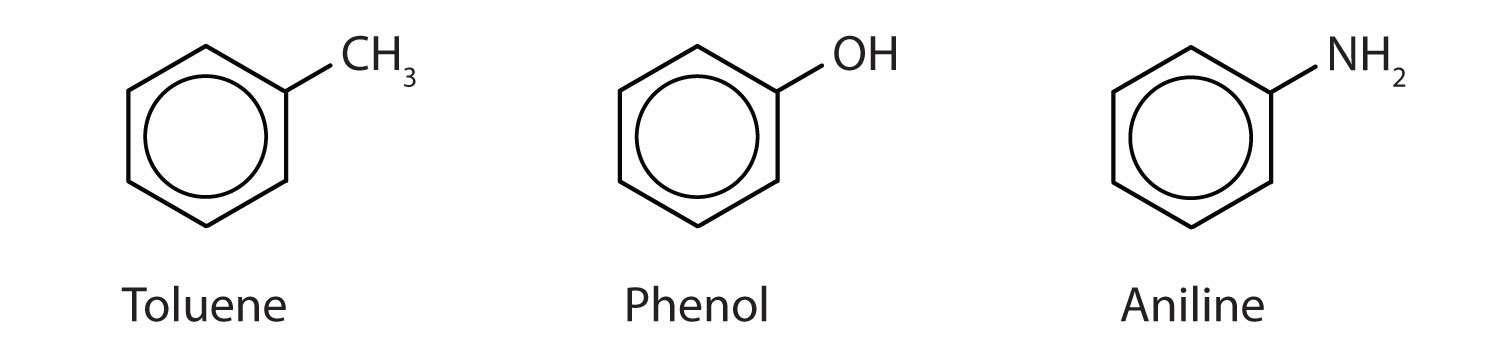
Although some compounds are referred to exclusively by IUPAC names, some are more frequently denoted by common names, as is indicated in Table \(\PageIndex{1}\).
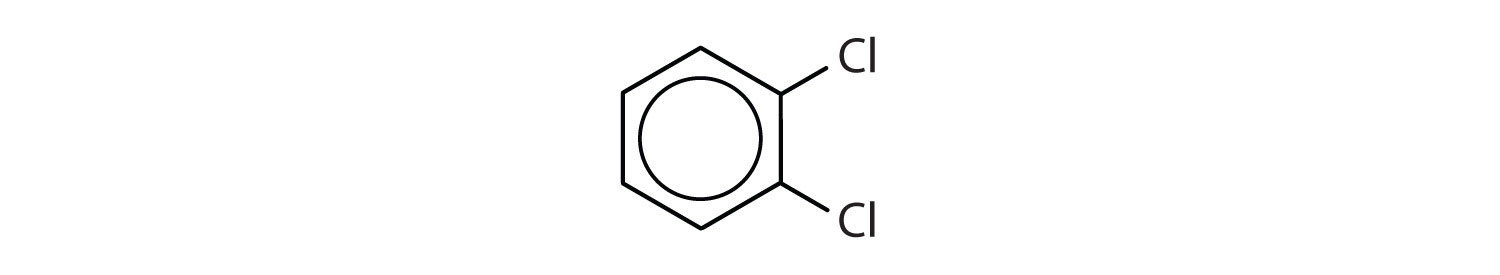
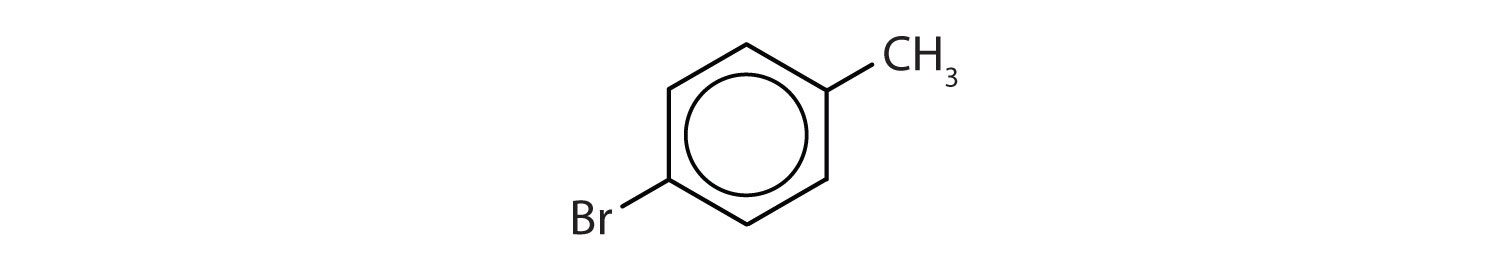
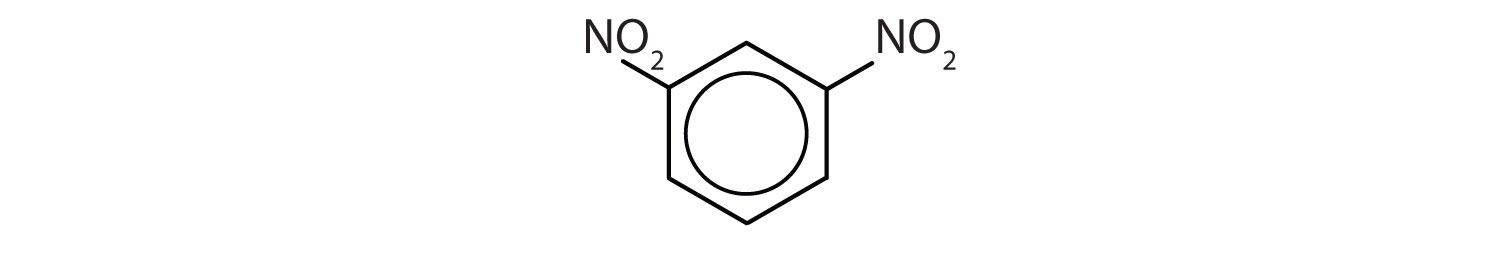
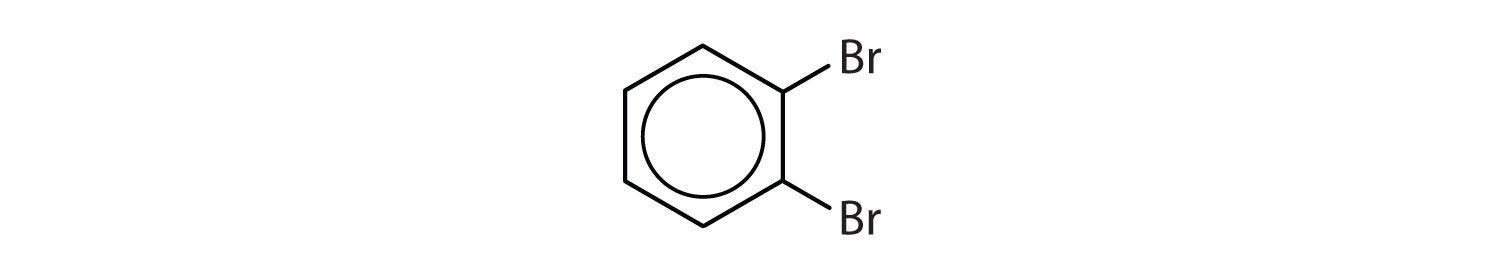
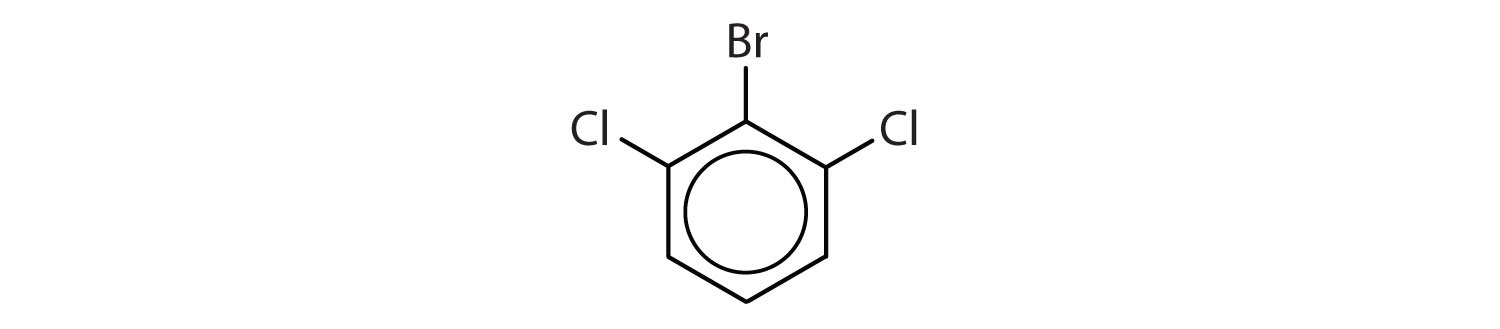
When there is more than one substituent, the corners of the hexagon are no longer equivalent, so we must designate the relative positions. There are three possible disubstituted benzenes, and we can use numbers to distinguish them (Figure \(\PageIndex{2}\)). We start numbering at the carbon atom to which one of the groups is attached and count toward the carbon atom that bears the other substituent group by the shortest path.
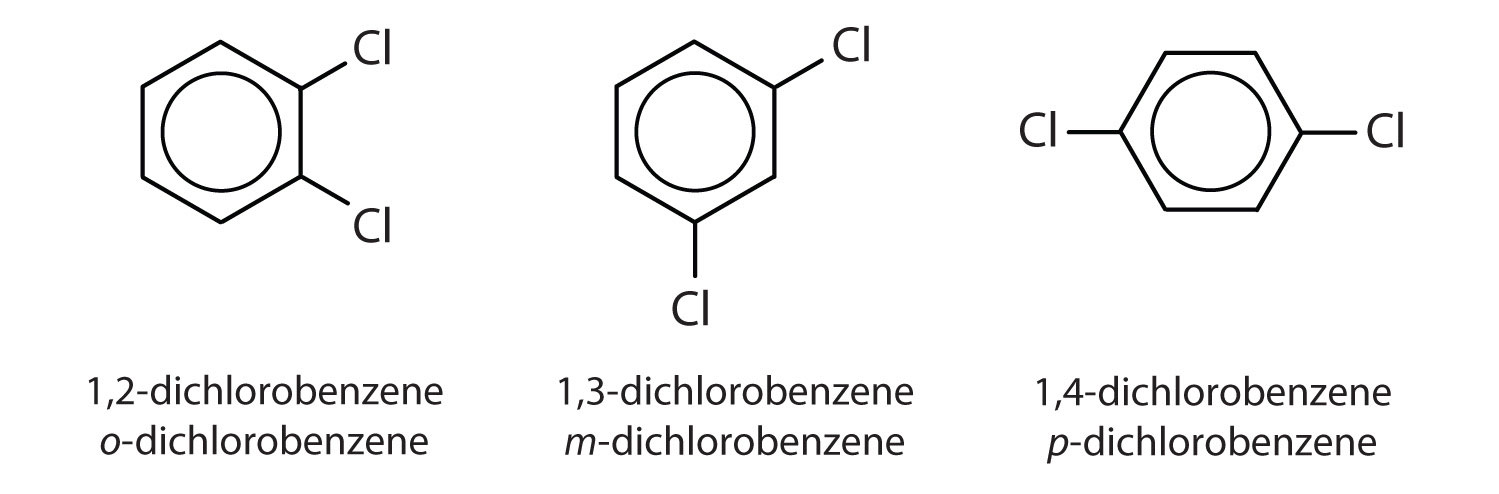
In Figure \(\PageIndex{2}\), common names are also used: the prefix ortho (o-) for 1,2-disubstitution, meta (m-) for 1,3-disubstitution, and para (p-) for 1,4-disubstitution. The substituent names are listed in alphabetical order. The first substituent is given the lowest number. When a common name is used, the carbon atom that bears the group responsible for the name is given the number 1:
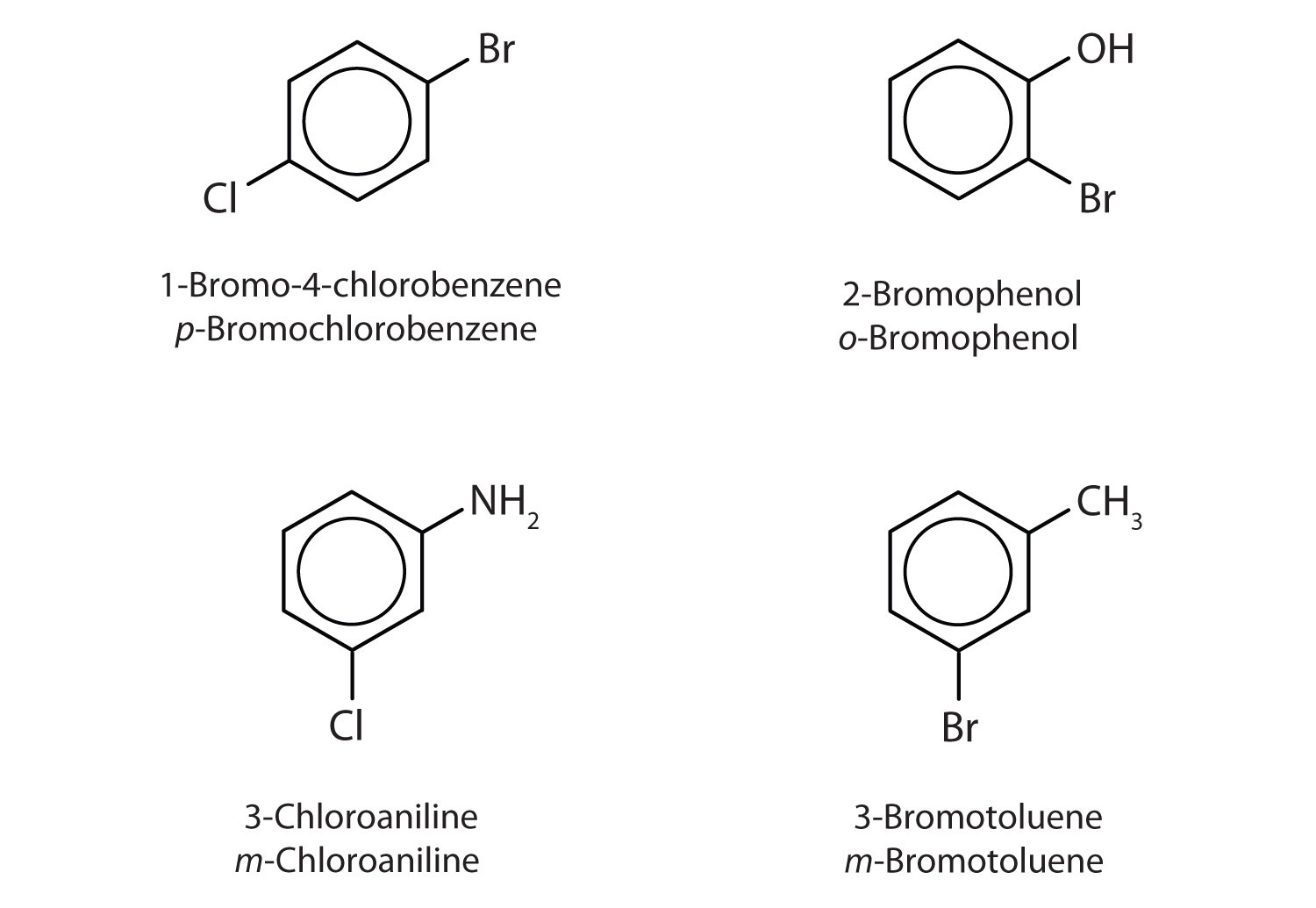
Name each compound using both the common name and the IUPAC name.
Solution
- The benzene ring has two chlorine atoms (dichloro) in the first and second positions. The compound is o-dichlorobenzene or 1,2-dichlorobenzene.
- The benzene ring has a methyl (CH3) group. The compound is therefore named as a derivative of toluene. The bromine atom is on the fourth carbon atom, counting from the methyl group. The compound is p-bromotoluene or 4-bromotoluene.
- The benzene ring has two nitro (NO2) groups in the first and third positions. It is m-dinitrobenzene or 1,3-dinitrobenzene.
Note: The nitro (NO2) group is a common substituent in aromatic compounds. Many nitro compounds are explosive, most notably 2,4,6-trinitrotoluene (TNT).
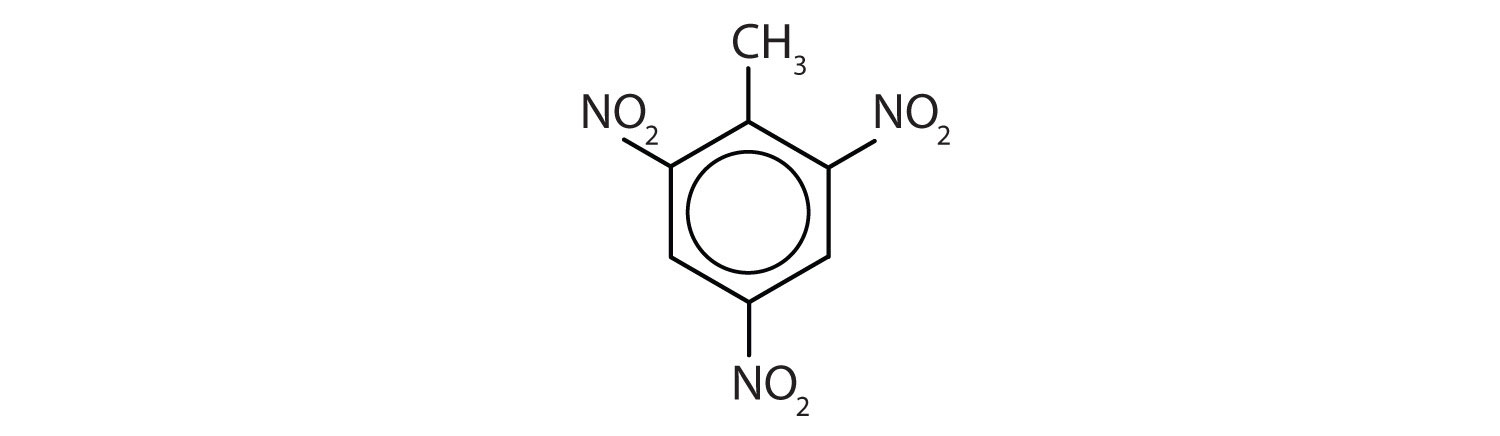
Name each compound using both the common name and the IUPAC name.
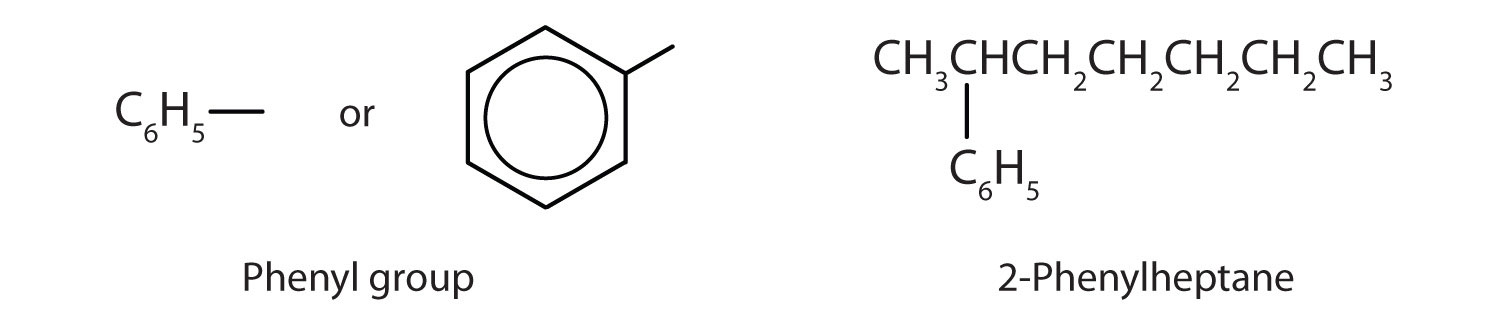
Sometimes an aromatic group is found as a substituent bonded to a nonaromatic entity or to another aromatic ring. The group of atoms remaining when a hydrogen atom is removed from an aromatic compound is called an aryl group. The most common aryl group is derived from benzene (C6H6) by removing one hydrogen atom (C6H5) and is called a phenyl group, from pheno, an old name for benzene.
Contributors and Attributions
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry