26.7: Organic Compounds Containing Functional Groups
- Page ID
- 24385
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Functional groups are atoms or small groups of atoms (two to four) that exhibit a characteristic reactivity. A particular functional group will almost always display its characteristic chemical behavior when it is present in a compound. Because of their importance in understanding organic chemistry, functional groups have characteristic names that often carry over in the naming of individual compounds incorporating specific groups
As we progress in our study of organic chemistry, it will become extremely important to be able to quickly recognize the most common functional groups, because they are the key structural elements that define how organic molecules react. For now, we will only worry about drawing and recognizing each functional group, as depicted by Lewis and line structures. Much of the remainder of your study of organic chemistry will be taken up with learning about how the different functional groups tend to behave in organic reactions.
Alcohols and Phenols
We have already seen the simplest possible example of an alcohol functional group in methanol. In the alcohol functional group, a carbon is single-bonded to an OH group (this OH group, by itself, is referred to as a hydroxyl). If the central carbon in an alcohol is bonded to only one other carbon, we call the group a primary alcohol. In secondary alcohols and tertiary alcohols, the central carbon is bonded to two and three carbons, respectively. Methanol, of course, is in class by itself in this respect.

Compounds in which an OH group is attached directly to an aromatic ring are designated ArOH and called phenols. Phenols differ from alcohols in that they are slightly acidic in water. They react with aqueous sodium hydroxide (NaOH) to form salts.
\[ArOH_{(aq)} + NaOH_{(aq)} \rightarrow ArONa_{(aq)} + H_2O\]
The parent compound, C6H5OH, is itself called phenol. (An old name, emphasizing its slight acidity, was carbolic acid.) Phenol is a white crystalline compound that has a distinctive (“hospital smell”) odor.
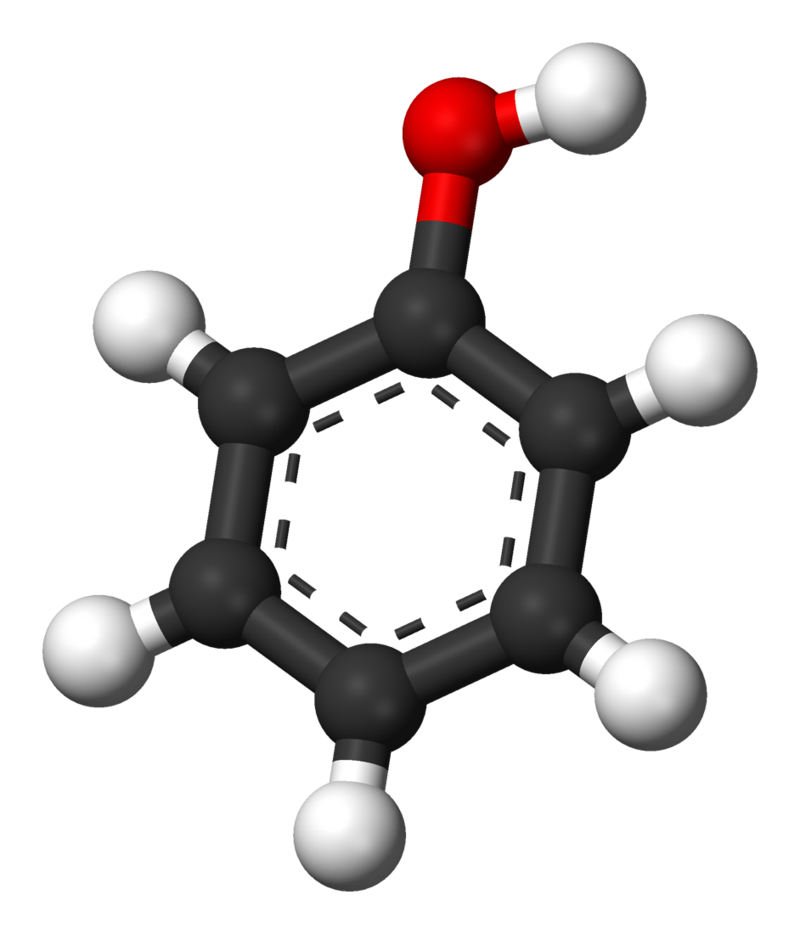

Preparation and Uses of Alcohols
There are two primary methods to make alcohols in the laboratory: Hydration of an alkene and hydrolysis of an alkyl halide.
Ethanol is manufactured by reacting ethene with steam. The catalyst used is solid silicon dioxide coated with phosphoric(V) acid. The reaction is reversible.

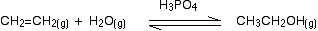
Some - but not all - other alcohols can be made by similar reactions. The catalyst used and the reaction conditions will vary from alcohol to alcohol. The reason that there is a problem with some alcohols is well illustrated with trying to make an alcohol from propene, CH3CH=CH2. In principle, there are two different alcohols which might be formed:
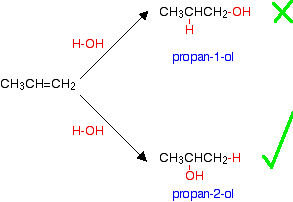
You might expect to get either propan-1-ol or propan-2-ol depending on which way around the water adds to the double bond. In practice what you get is propan-2-ol. If you add a molecule H-X across a carbon-carbon double bond, the hydrogen nearly always gets attached to the carbon with the most hydrogens on it already - in this case the CH2 rather than the CH. The effect of this is that there are bound to be some alcohols which it is impossible to make by reacting alkenes with steam because the addition would be the wrong way around.
The other common method to make alcholes is a substitution reaction, the halogen atom is replaced by an -OH group to give an alcohol. For example:
![]()
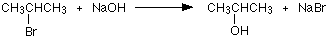
Or, as an ionic equation:
![]()
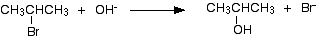
In this example, 2-bromopropane is converted into propan-2-ol. The halogenoalkane is heated under reflux with a solution of sodium or potassium hydroxide. Heating under reflux means heating with a condenser placed vertically in the flask to prevent loss of volatile substances from the mixture.
The solvent is usually a 50/50 mixture of ethanol and water, because everything will dissolve in that. The halogenoalkane is insoluble in water. If you used water alone as the solvent, the halogenoalkane and the sodium hydroxide solution wouldn't mix and the reaction could only happen where the two layers met.
Ethanol is usually sold as industrial methylated spirits, which is ethanol with a small quantity of methanol and possibly some color added. Because methanol is poisonous, industrial methylated spirits are unfit to drink, allowing purchasers to avoid the high taxes levied on alcoholic drinks. Ethanol burns to produce carbon dioxide and water, as shown in the equation below, and can be used as a fuel in its own right or in mixtures with petrol (gasoline). "Gasohol" is a petrol/ethanol mixture containing approximately 10–20% ethanol. Because ethanol can be produced by fermentation, this is a useful method for countries without an oil industry to reduce the amount of petrol imports.
\[CH_3CH_2OH +3O_2 \rightarrow 2CO_2 + 3H_2O\]
Ethanol is widely used as a solvent. It is relatively safe and can be used to dissolve many organic compounds that are insoluble in water. It is used, for example, in many perfumes and cosmetics.
Methanol also burns to form carbon dioxide and water:
\[2CH_3OH +3O_2 \rightarrow 2CO_2 + 4H_2O\]
It can be used a a petrol additive to improve combustion, and its use as a fuel in its own right is under investigation. Furthremore, most methanol is used to make other compounds, for example, methanal (formaldehyde), ethanoic acid, and methyl esters of various acids. In most cases, these are then converted into further products.
Phenols are widely used as antiseptics (substances that kill microorganisms on living tissue) and as disinfectants (substances intended to kill microorganisms on inanimate objects such as furniture or floors). The first widely used antiseptic was phenol. Joseph Lister used it for antiseptic surgery in 1867. Phenol is toxic to humans, however, and can cause severe burns when applied to the skin. In the bloodstream, it is a systemic poison—that is, one that is carried to and affects all parts of the body. Its severe side effects led to searches for safer antiseptics, a number of which have been found.
Ethers
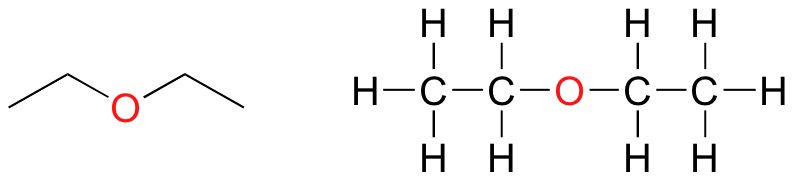
In an ether functional group, a central oxygen is bonded to two carbons. Below are the line and Lewis structures of diethyl ether, a common laboratory solvent and also one of the first medical anaesthesia agents.
Preparation and Uses of Ethers
Acid-catalyzed dehydration of small 1º-alcohols constitutes a specialized method of preparing symmetrical ethers. As shown in the following two equations, the success of this procedure depends on the temperature. At 110º to 130 ºC an SN2 reaction of the alcohol conjugate acid leads to an ether product. At higher temperatures (over 150 ºC) an E2 elimination takes place.
| 2 CH3CH2-OH + H2SO4 | 130 ºC |
CH3CH2-O-CH2CH3 + H2O |
| CH3CH2-OH + H2SO4 | 150 ºC |
CH2=CH2 + H2O |
In this reaction alcohol has to be used in excess and the temperature has to be maintained around 413 K. If alcohol is not used in excess or the temperature is higher, the alcohol will preferably undergo dehydration to yield alkene.
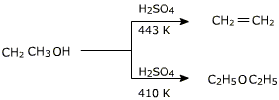
If ethanol is dehydrated to ethene in presence of sulfuric acid at 433 K but as 410 K, ethoxyethane is the main product. The dehydration of secondary and tertiary alcohols to get corresponding ethers is unsuccessful as alkenes are formed easily in these reactions.
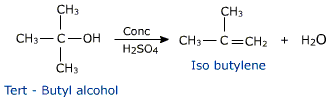
This reaction cannot be employed to prepare unsymmetrical ethers. It is because a mixture of products is likely to be obtained.
Aldehydes and Ketones
There are a number of functional groups that contain a carbon-oxygen double bond, which is commonly referred to as a carbonyl. Ketones and aldehydes are two closely related carbonyl-based functional groups that react in very similar ways. In a ketone, the carbon atom of a carbonyl is bonded to two other carbons. In an aldehyde, the carbonyl carbon is bonded on one side to a hydrogen, and on the other side to a carbon. The exception to this definition is formaldehyde, in which the carbonyl carbon has bonds to two hydrogens.
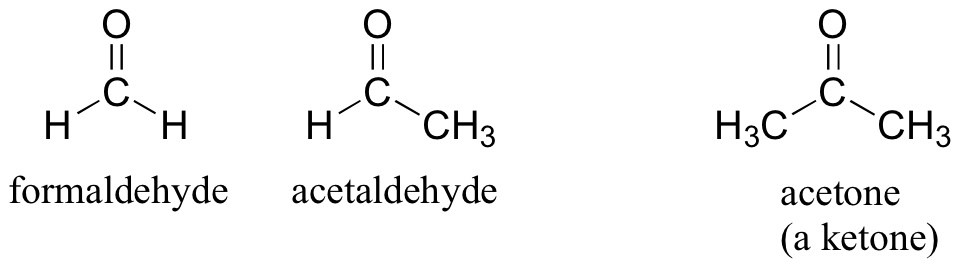
Preparation and Uses of Aldehydes and Ketones
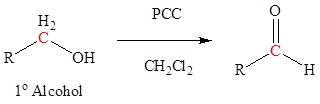
Aldehydes and ketones can be prepared using a wide variety of reactions. Although these reactions are discussed in greater detail in other sections, they are listed here as a summary and to help with planning multistep synthetic pathways. A common way to synthesize aldehydes is the ixidation of 1o alcohols to form aldehydes
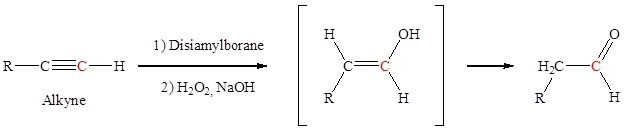
Hydration of an alkyne to form aldehydes via an addition reaction of a hydroxyl group to an alkyne forms an aldehyde. The addition of a hydroxyl group to an alkyne causes tautomerization which subsequently forms a carbonyl.
Carboxylic acids
If a carbonyl carbon is bonded on one side to a carbon (or hydrogen) and on the other side to a heteroatom (in organic chemistry, this term generally refers to oxygen, nitrogen, sulfur, or one of the halogens), the functional group is considered to be one of the ‘carboxylic acid derivatives’, a designation that describes a grouping of several functional groups. The eponymous member of this grouping is the carboxylic acid functional group, in which the carbonyl is bonded to a hydroxyl (OH) group.
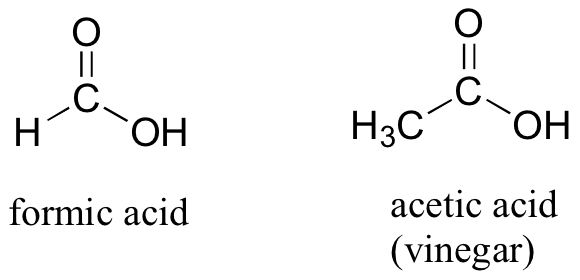
As the name implies, carboxylic acids are acidic, meaning that they are readily deprotonated to form the conjugate base form, called a carboxylate (much more about carboxylic acids in the acid-base chapter!).
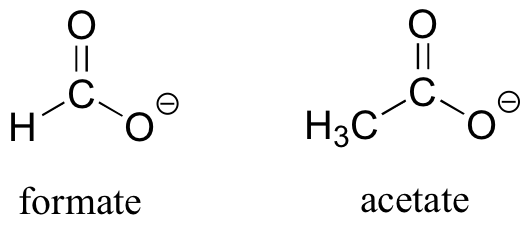
Preparation and Uses of Carboxylic Acids
The oxidation of aldehydes or primary alcohols forms carboxylic acids:

In the presence of an oxidizing agent, ethanol is oxidized to acetaldehyde, which is then oxidized to acetic acid.
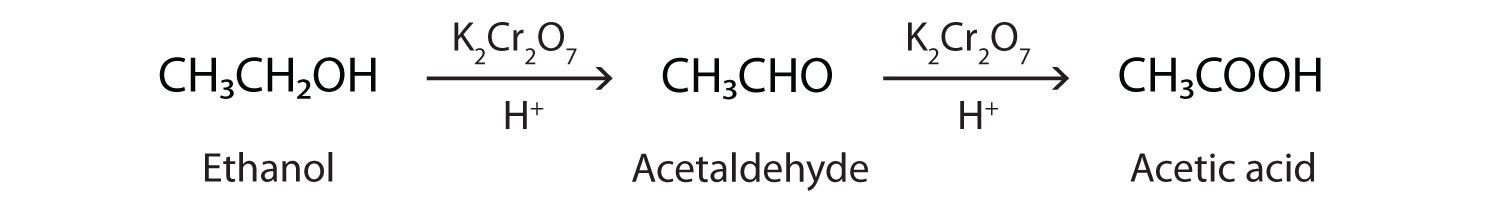
This process also occurs in the liver, where enzymes catalyze the oxidation of ethanol to acetic acid.
\(\mathrm{CH_3CH_2OH \underset{oxidizing\: agent}{\xrightarrow{alcohol\: dehydrogenase}} CH_3CHO \underset{oxidizing\: agent}{\xrightarrow{alcohol\: dehydrogenase}} CH_3COOH\%}\)
Acetic acid can be further oxidized to carbon dioxide and water.
Esters
In esters, the carbonyl carbon is bonded to an oxygen which is itself bonded to another carbon. Another way of thinking of an ester is that it is a carbonyl bonded to an alcohol. Thioesters are similar to esters, except a sulfur is in place of the oxygen.
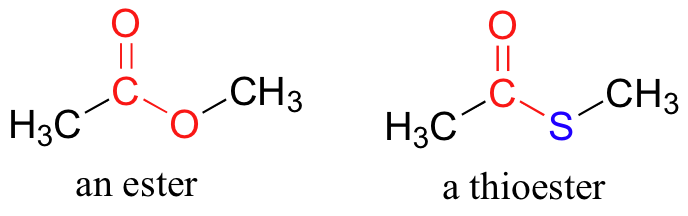
Preparation and Uses of Esters
Some esters can be prepared by esterification, a reaction in which a carboxylic acid and an alcohol, heated in the presence of a mineral acid catalyst, form an ester and water:
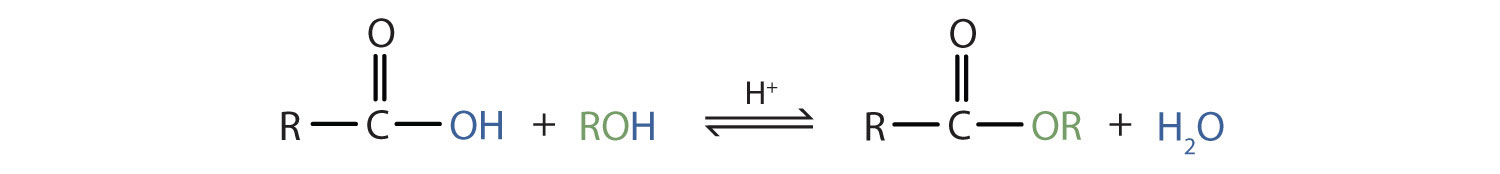
The reaction is reversible. As a specific example of an esterification reaction, butyl acetate can be made from acetic acid and 1-butanol.
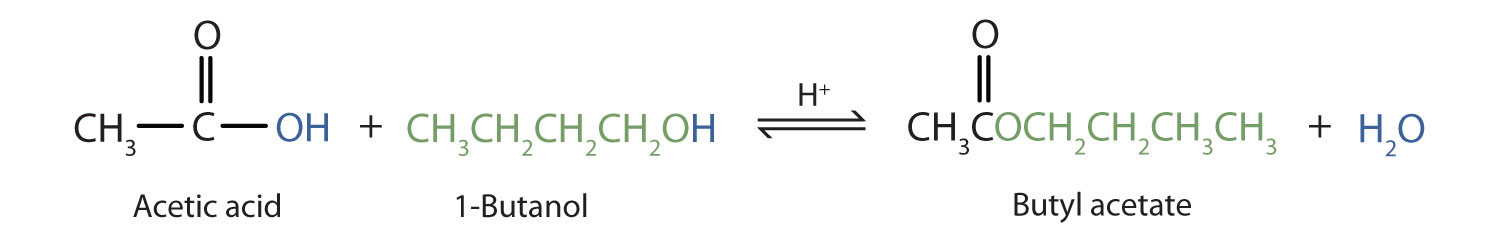
Esters are common solvents. Ethyl acetate is used to extract organic solutes from aqueous solutions—for example, to remove caffeine from coffee. It also is used to remove nail polish and paint. Cellulose nitrate is dissolved in ethyl acetate and butyl acetate to form lacquers. The solvent evaporates as the lacquer “dries,” leaving a thin film on the surface. High boiling esters are used as softeners (plasticizers) for brittle plastics.
Amides
In amides, the carbonyl carbon is bonded to a nitrogen. The nitrogen in an amide can be bonded either to hydrogens, to carbons, or to both. Another way of thinking of an amide is that it is a carbonyl bonded to an amine.
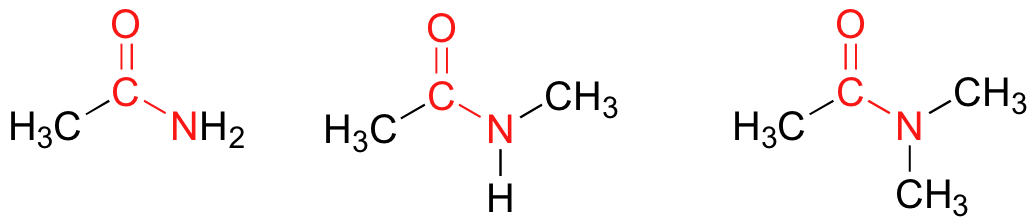
Preparation and Uses of Amides
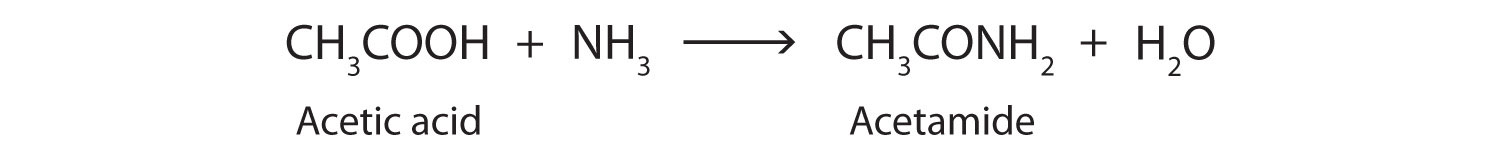
The addition of ammonia (NH3) to a carboxylic acid forms an amide, but the reaction is very slow in the laboratory at room temperature. Water molecules are split out, and a bond is formed between the nitrogen atom and the carbonyl carbon atom.
In living cells, amide formation is catalyzed by enzymes. Proteins are polyamides; they are formed by joining amino acids into long chains. In proteins, the amide functional group is called a peptide bond.
With the exception of formamide (HCONH2), which is a liquid, all simple amides are solids (Table \(\PageIndex{1}\)). The lower members of the series are soluble in water, with borderline solubility occurring in those that have five or six carbon atoms. Like the esters, solutions of amides in water usually are neutral—neither acidic nor basic.
| Condensed Structural Formula | Name | Melting Point (°C) | Boiling Point (°C) | Solubility in Water |
|---|---|---|---|---|
| HCONH2 | formamide | 2 | 193 | soluble |
| CH3CONH2 | acetamide | 82 | 222 | soluble |
| CH3CH2CONH2 | propionamide | 81 | 213 | soluble |
| CH3CH2CH2CONH2 | butyramide | 115 | 216 | soluble |
| C6H5CONH2 | benzamide | 132 | 290 | slightly soluble |
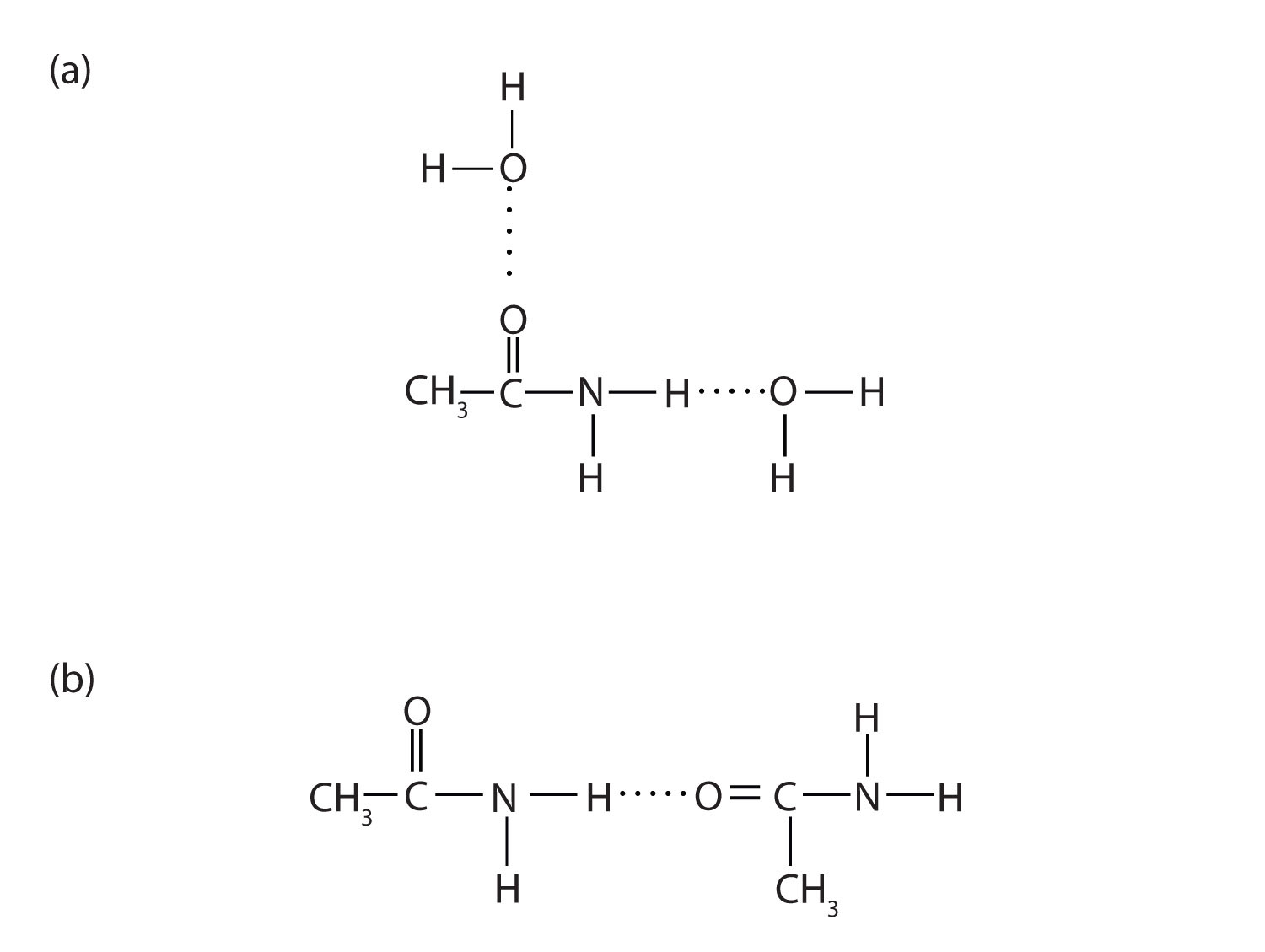
The amides generally have high boiling points and melting points. These characteristics and their solubility in water result from the polar nature of the amide group and hydrogen bonding (Figure \(\PageIndex{1}\)). (Similar hydrogen bonding plays a critical role in determining the structure and properties of proteins, deoxyribonucleic acid [DNA], ribonucleic acid [RNA], and other giant molecules so important to life processes.
Amines
Ammonia is the simplest example of a functional group called amines. Just as there are primary, secondary, and tertiary alcohols, there are primary, secondary, and tertiary amines.
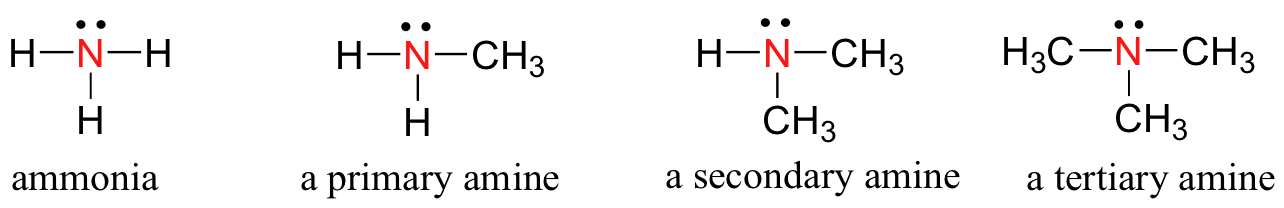
One of the most important properties of amines is that they are basic, and are readily protonated to form ammonium cations.
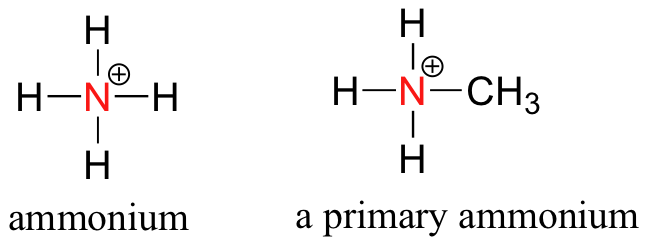
Preparation and Uses of Amines
The halogenoalkane is heated with a concentrated solution of ammonia in ethanol. The reaction is carried out in a sealed tube. You couldn't heat this mixture under reflux, because the ammonia would simply escape up the condenser as a gas. We'll talk about the reaction using 1-bromoethane as a typical halogenoalkane. You get a mixture of amines formed together with their salts. The reactions happen one after another.
The reaction happens in two stages. In the first stage, a salt is formed - in this case, ethylammonium bromide. This is just like ammonium bromide, except that one of the hydrogens in the ammonium ion is replaced by an ethyl group.
\[ CH_3CH_2Br + NH_3 \rightarrow CH_3CH_2NH_3^+Br^-\]
There is then the possibility of a reversible reaction between this salt and excess ammonia in the mixture.
\[ CH_3CH_2NH_3^+Br^- + NH_3 \rightleftharpoons CH_3CH_2NH_2 + NH_4^+ Br^-\]
The ammonia removes a hydrogen ion from the ethylammonium ion to leave a primary amine - ethylamine. The more ammonia there is in the mixture, the more the forward reaction is favored.
The reaction does not stop at a primary amine. The ethylamine also reacts with bromoethane - in the same two stages as before. In the first stage, you get a salt formed - this time, diethylammonium bromide. Think of this as ammonium bromide with two hydrogens replaced by ethyl groups.

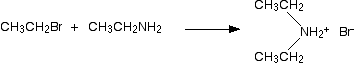
There is again the possibility of a reversible reaction between this salt and excess ammonia in the mixture.


The ammonia removes a hydrogen ion from the diethylammonium ion to leave a secondary amine - diethylamine. A secondary amine is one which has two alkyl groups attached to the nitrogen. The reaction does not stop! The diethylamine also reacts with bromoethane - in the same two stages as before. In the first stage, you get triethylammonium bromide.

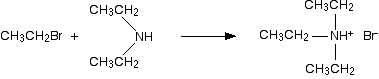
There is again the possibility of a reversible reaction between this salt and excess ammonia in the mixture.


The ammonia removes a hydrogen ion from the triethylammonium ion to leave a tertiary amine - triethylamine. A tertiary amine is one which has three alkyl groups attached to the nitrogen.
The final stage! The triethylamine reacts with bromoethane to give tetraethylammonium bromide - a quaternary ammonium salt (one in which all four hydrogens have been replaced by alkyl groups).
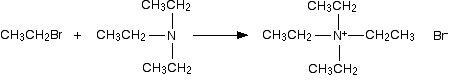
This time there isn't any hydrogen left on the nitrogen to be removed. The reaction stops here.
Molecules with Multiple Functional Groups
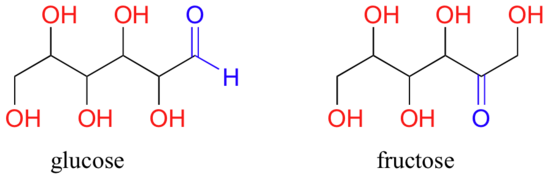
A single compound often contains several functional groups. The six-carbon sugar molecules glucose and fructose, for example, contain aldehyde and ketone groups, respectively, and both contain five alcohol groups (a compound with several alcohol groups is often referred to as a ‘polyol’).
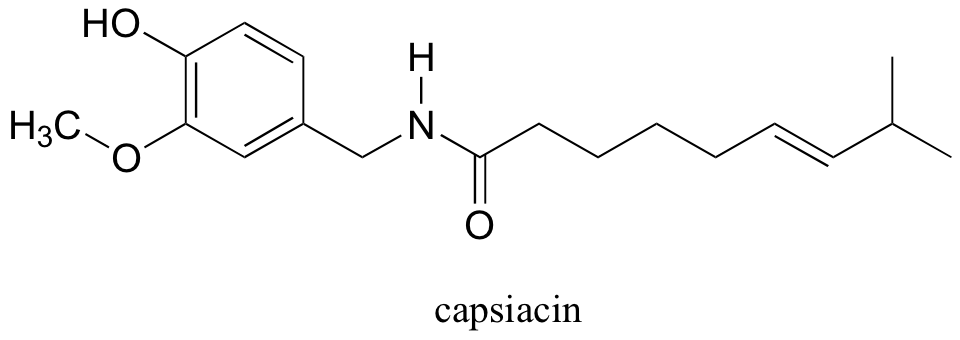
Capsaicin, the compound responsible for the heat in hot peppers, contains phenol, ether, amide, and alkene functional groups.
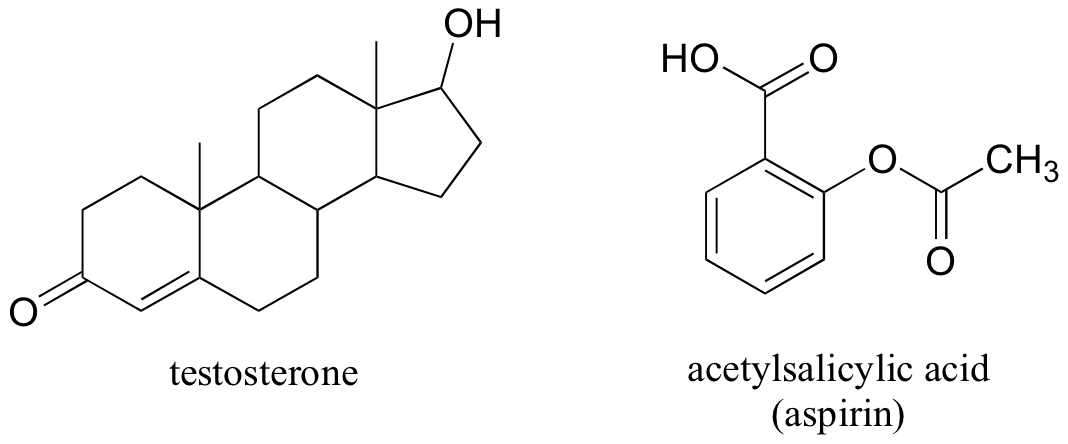
The male sex hormone testosterone contains ketone, alkene, and secondary alcohol groups, while acetylsalicylic acid (aspirin) contains aromatic, carboxylic acid, and ester groups.
While not in any way a complete list, this section has covered most of the important functional groups that we will encounter in biological and laboratory organic chemistry. The table on the inside back cover provides a summary of all of the groups listed in this section, plus a few more that will be introduced later in the text.
Identify the functional groups in the following organic compounds. State whether alcohols and amines are primary, secondary, or tertiary.
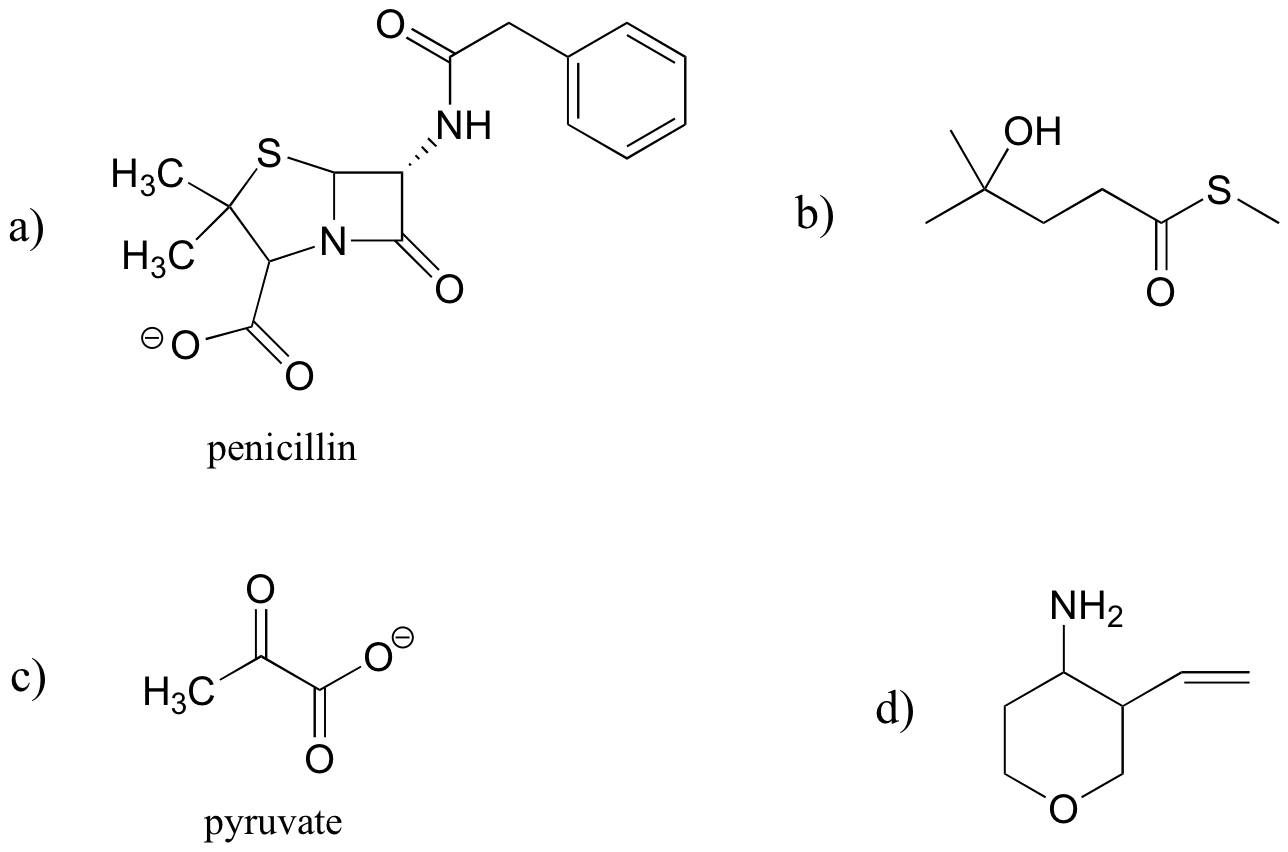
Draw one example each (there are many possible correct answers) of compounds fitting the descriptions below, using line structures. Be sure to designate the location of all non-zero formal charges. All atoms should have complete octets (phosphorus may exceed the octet rule).
- a compound with molecular formula C6H11NO that includes alkene, secondary amine, and primary alcohol functional groups
- an ion with molecular formula C3H5O6P 2- that includes aldehyde, secondary alcohol, and phosphate functional groups.
- A compound with molecular formula C6H9NO that has an amide functional group, and does not have an alkene group.
Contributors and Attributions
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

