23.1: General Properties of Transition Metals
- Page ID
- 24341
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)We have daily contact with many transition metals. Iron occurs everywhere—from the rings in your spiral notebook and the cutlery in your kitchen to automobiles, ships, buildings, and in the hemoglobin in your blood. Titanium is useful in the manufacture of lightweight, durable products such as bicycle frames, artificial hips, and jewelry. Chromium is useful as a protective plating on plumbing fixtures and automotive detailing.
Transition metals are defined as those elements that have (or readily form) partially filled d orbitals. As shown in Figure \(\PageIndex{2}\), the d-block elements in groups 3–11 are transition elements. The f-block elements, also called inner transition metals (the lanthanides and actinides), also meet this criterion because the d orbital is partially occupied before the f orbitals. The d orbitals fill with the copper family (group 11); for this reason, the next family (group 12) are technically not transition elements. However, the group 12 elements do display some of the same chemical properties and are commonly included in discussions of transition metals. Some chemists do treat the group 12 elements as transition metals.

The d-block elements are divided into the first transition series (the elements Sc through Cu), the second transition series (the elements Y through Ag), and the third transition series (the element La and the elements Hf through Au). Actinium, Ac, is the first member of the fourth transition series, which also includes Rf through Rg.
The f-block elements are the elements Ce through Lu, which constitute the lanthanide series (or lanthanoid series), and the elements Th through Lr, which constitute the actinide series (or actinoid series). Because lanthanum behaves very much like the lanthanide elements, it is considered a lanthanide element, even though its electron configuration makes it the first member of the third transition series. Similarly, the behavior of actinium means it is part of the actinide series, although its electron configuration makes it the first member of the fourth transition series
Properties and Trends in Transition Metals
The elements of the second and third rows of the Periodic Table show gradual changes in properties across the table from left to right as expected. Electrons in the outer shells of the atoms of these elements have little shielding effects resulting in an increase in effective nuclear charge due to the addition of protons in the nucleus. Consequently, the effects on atomic properties are: smaller atomic radius, increased first ionization energy, enhanced electronegativity and more nonmetallic character. This trend continues until one reaches calcium (Z=20). There is an abrupt break at this point. The next ten elements called the first transition series are remarkably similar in their physical and chemical properties. This general similarity in properties has been explained in terms of their relatively small difference in effective nuclear charge over the series. This occurs because each additional electron enters the penultimate 3d shell providing an effective shield between the nucleus and the outer 4s shell.
Thus, the transition elements can be defined as those in which the d electron shells are being filled and so we generally ignore Sc and Zn where Sc(III) is d0 and Zn(II) is d10.
It is useful, at the beginning, to identify the physical and chemical properties of transition elements which differ from main group elements (s-block). Properties of transition elements include:
- have large charge/radius ratio;
- are hard and have high densities;
- have high melting and boiling points;
- form compounds which are often paramagnetic;
- show variable oxidation states;
- form coloured ions and compounds;
- form compounds with profound catalytic activity;
- form stable complexes.
| Element | Group | density /g cm-3 |
m. p. / °C |
b.p. / °C |
radius / pm |
free atom configuration |
ionization energy / kJ mol-1 |
Uses |
|---|---|---|---|---|---|---|---|---|
| Sc | 3 | 2.99 | 1541 | 2831 | 164 | [Ar] 3d14s2 | 631 | |
| Ti | 4 | 4.50 | 1660 | 3287 | 147 | [Ar]3d24s2 | 658 | -engines/aircraft industry-density is 60% of iron |
| V | 5 | 5.96 | 1890 | 3380 | 135 | [Ar]3d34s2 | 650 | -stainless steel, 19% Cr, 9% Ni the rest Fe |
| Cr | 6 | 7.20 | 1857 | 2670 | 129 | [Ar]3d54s1 | 653 | -alloys eg with C steel, the most significant use |
| Mn | 7 | 7.20 | 1244 | 1962 | 137 | [Ar]3d54s2 | 717 | -alloys eg with Cu |
| Fe | 8 | 7.86 | 1535 | 2750 | 126 | [Ar]3d64s2 | 759 | -alloys eg with C steel, the most significant use |
| Co | 9 | 8.90 | 1495 | 2870 | 125 | [Ar]3d74s2 | 758 | -alloys eg with Cr and W for hardened drill bits |
| Ni | 10 | 8.90 | 1455 | 2730 | 125 | [Ar]3d84s2 | 737 | -alloys Fe/Ni armor plating, resists corrosion |
| Cu | 11 | 8.92 | 1083 | 2567 | 128 | [Ar]3d104s1 | 746 | -high electrical conductivity (2nd to Ag), wiring |
| Zn | 12 | 7.14 | 420 | 907 | 137 | [Ar]3d104s2 | 906 |
Densities and Metallic Radii
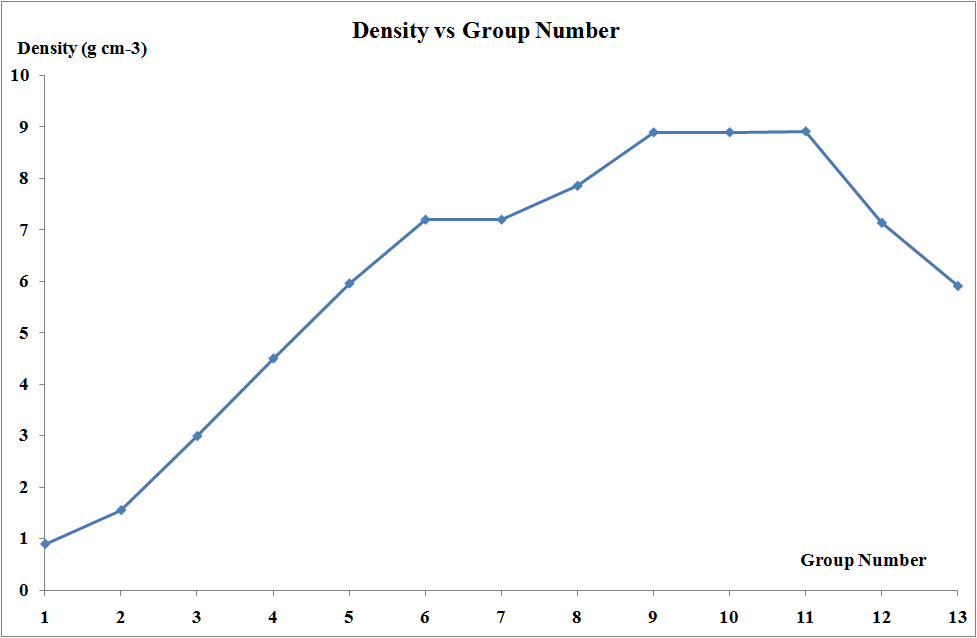
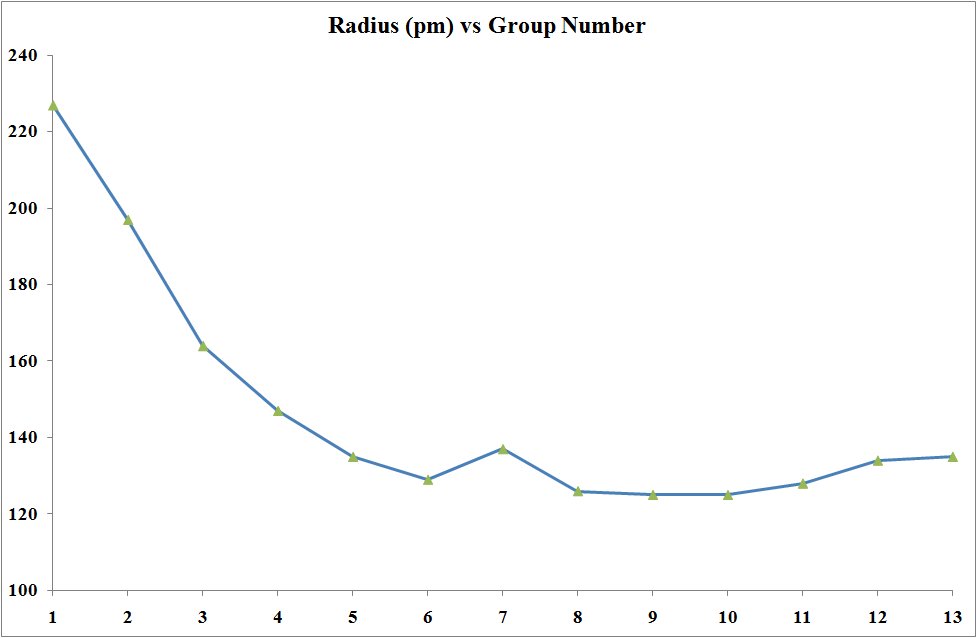
The transition elements are much denser than the s-block elements and show a gradual increase in density from scandium to copper. This trend in density can be explained by the small and irregular decrease in metallic radii coupled with the relative increase in atomic mass.
Melting and Boiling points
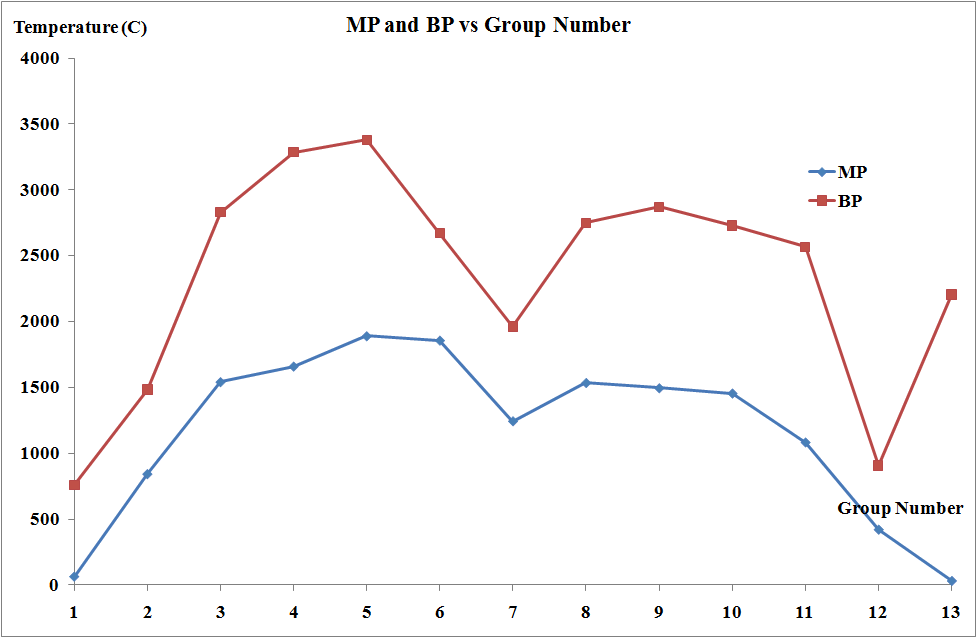
The melting points and the molar enthalpies of fusion of the transition metals are both high in comparison to main group elements. This arises from strong metallic bonding in transition metals which occurs due to delocalization of electrons facilitated by the availability of both d and s electrons.
Ionization Energies
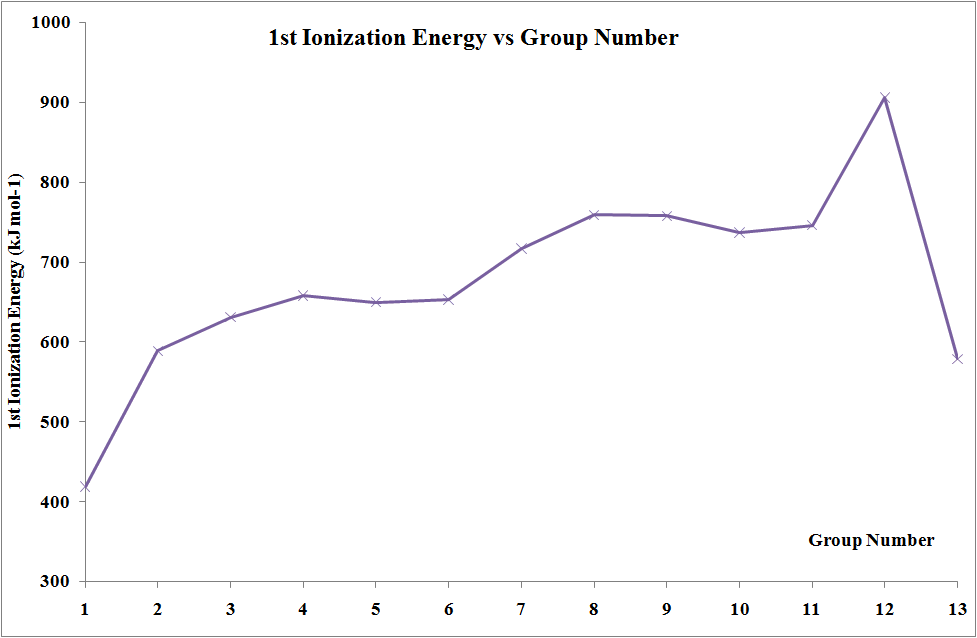
In moving across the series of metals from scandium to zinc a small change in the values of the first and second ionization energies is observed. This is due to the build-up of electrons in the immediately underlying d-sub-shells that efficiently shields the 4s electrons from the nucleus and minimizing the increase in effective nuclear charge \(Z_{eff}\) from element to element. The increases in third and fourth ionization energy values are more rapid. However, the trends in these values show the usual discontinuity half way along the series. The reason is that the five d electrons are all unpaired, in singly occupied orbitals. When the sixth and subsequent electrons enter, the electrons have to share the already occupied orbitals resulting in inter-electron repulsions, which would require less energy to remove an electron. Hence, the third ionization energy curve for the last five elements is identical in shape to the curve for the first five elements, but displaced upwards by 580 kJ mol-1.
Oxidation States
Transition metals can form compounds with a wide range of oxidation states. Some of the observed oxidation states of the elements of the first transition series are shown in Figure \(\PageIndex{5}\). As we move from left to right across the first transition series, we see that the number of common oxidation states increases at first to a maximum towards the middle of the table, then decreases. The values in the table are typical values; there are other known values, and it is possible to synthesize new additions. For example, in 2014, researchers were successful in synthesizing a new oxidation state of iridium (9+).
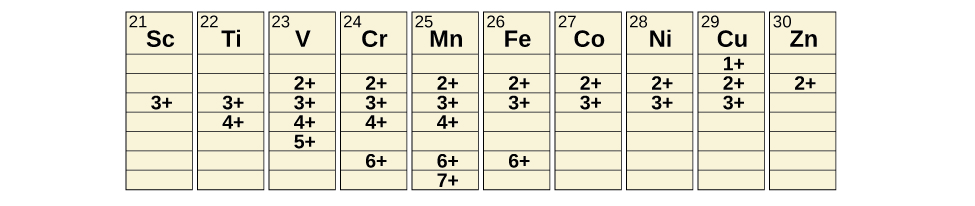
For the elements scandium through manganese (the first half of the first transition series), the highest oxidation state corresponds to the loss of all of the electrons in both the s and d orbitals of their valence shells. The titanium(IV) ion, for example, is formed when the titanium atom loses its two 3d and two 4s electrons. These highest oxidation states are the most stable forms of scandium, titanium, and vanadium. However, it is not possible to continue to remove all of the valence electrons from metals as we continue through the series. Iron is known to form oxidation states from 2+ to 6+, with iron(II) and iron(III) being the most common. Most of the elements of the first transition series form ions with a charge of 2+ or 3+ that are stable in water, although those of the early members of the series can be readily oxidized by air.
The elements of the second and third transition series generally are more stable in higher oxidation states than are the elements of the first series. In general, the atomic radius increases down a group, which leads to the ions of the second and third series being larger than are those in the first series. Removing electrons from orbitals that are located farther from the nucleus is easier than removing electrons close to the nucleus. For example, molybdenum and tungsten, members of group 6, are limited mostly to an oxidation state of 6+ in aqueous solution. Chromium, the lightest member of the group, forms stable Cr3+ ions in water and, in the absence of air, less stable Cr2+ ions. The sulfide with the highest oxidation state for chromium is Cr2S3, which contains the Cr3+ ion. Molybdenum and tungsten form sulfides in which the metals exhibit oxidation states of 4+ and 6+.
Electronic Configurations
The electronic configuration of the atoms of the first row transition elements are basically the same. It can be seen in the Table above that there is a gradual filling of the 3d orbitals across the series starting from scandium. This filling is, however, not regular, since at chromium and copper the population of 3d orbitals increase by the acquisition of an electron from the 4s shell. This illustrates an important generalization about orbital energies of the first row transition series. At chromium, both the 3d and 4s orbitals are occupied, but neither is completely filled in preference to the other. This suggests that the energies of the 3d and 4s orbitals are relatively close for atoms in this row.
In the case of copper, the 3d level is full, but only one electron occupies the 4s orbital. This suggests that in copper the 3d orbital energy is lower than the 4s orbital. Thus the 3d orbital energy has passed from higher to lower as we move across the period from potassium to zinc. However, the whole question of preference of an atom to adopt a particular electronic configuration is not determined by orbital energy alone. In chromium it can be shown that the 4s orbital energy is still below the 3d which suggests a configuration [Ar] 3d44s2. However due to the effect of electronic repulsion between the outer electrons the actual configuration becomes [Ar]3d54s1 where all the electrons in the outer orbitals are unpaired. It should be remembered that the factors that determine electronic configuration in this period are indeed delicately balanced.
| Redox Couple | E°/V |
| Mn2+(aq.)/Mn(s) | -1.18 |
| H+(aq.)/H2(g) | 0.00 |
This shows that elemental Mn is a stronger reductant than molecular hydrogen and hence should be able to displace hydrogen gas from 1 mol dm-3 hydrochloric acid.
Review how to write electron configurations, covered in the chapter on electronic structure and periodic properties of elements. Recall that for the transition and inner transition metals, it is necessary to remove the s electrons before the d or f electrons. Then, for each ion, give the electron configuration:
- cerium(III)
- lead(II)
- Ti2+
- Am3+
- Pd2+
For the examples that are transition metals, determine to which series they belong.
Solution
For ions, the s-valence electrons are lost prior to the d or f electrons.
- Ce3+[Xe]4f1; Ce3+ is an inner transition element in the lanthanide series.
- Pb2+[Xe]6s25d104f14; the electrons are lost from the p orbital. This is a main group element.
- titanium(II) [Ar]3d2; first transition series
- americium(III) [Rn]5f6; actinide
- palladium(II) [Kr]4d8; second transition series
Check Your Learning Give an example of an ion from the first transition series with no d electrons.
Answer
V5+ is one possibility. Other examples include Sc3+, Ti4+, Cr6+, and Mn7+.
Chemical Reactivity
Transition metals demonstrate a wide range of chemical behaviors. As can be seen from their reduction potentials (Table P1), some transition metals are strong reducing agents, whereas others have very low reactivity. For example, the lanthanides all form stable 3+ aqueous cations. The driving force for such oxidations is similar to that of alkaline earth metals such as Be or Mg, forming Be2+ and Mg2+. On the other hand, materials like platinum and gold have much higher reduction potentials. Their ability to resist oxidation makes them useful materials for constructing circuits and jewelry.
Ions of the lighter d-block elements, such as Cr3+, Fe3+, and Co2+, form colorful hydrated ions that are stable in water. However, ions in the period just below these (Mo3+, Ru3+, and Ir2+) are unstable and react readily with oxygen from the air. The majority of simple, water-stable ions formed by the heavier d-block elements are oxyanions such as \(\ce{MoO4^2-}\) and \(\ce{ReO4-}\).
Ruthenium, osmium, rhodium, iridium, palladium, and platinum are the "platinum metals". With difficulty, they form simple cations that are stable in water, and, unlike the earlier elements in the second and third transition series, they do not form stable oxyanions.
Both the d- and f-block elements react with nonmetals to form binary compounds; heating is often required. These elements react with halogens to form a variety of halides ranging in oxidation state from 1+ to 6+. On heating, oxygen reacts with all of the transition elements except palladium, platinum, silver, and gold. The oxides of these latter metals can be formed using other reactants, but they decompose upon heating. The f-block elements, the elements of group 3, and the elements of the first transition series except copper react with aqueous solutions of acids, forming hydrogen gas and solutions of the corresponding salts.
Which is the strongest oxidizing agent in acidic solution: dichromate ion, which contains chromium(VI), permanganate ion, which contains manganese(VII), or titanium dioxide, which contains titanium(IV)?
Solution
First, we need to look up the reduction half reactions (Table P1) for each oxide in the specified oxidation state:
\[\ce{Cr2O7^2- + 14H+ + 6e- ⟶ 2Cr^3+ + 7H2O} \hspace{20px} \mathrm{+1.33\: V}\]
\[\ce{MnO4- + 8H+ + 5e- ⟶ Mn^2+ + H2O} \hspace{20px} \mathrm{+1.51\: V}\]
\[\ce{TiO2 + 4H+ + 2e- ⟶ Ti^2+ + 2H2O} \hspace{20px} \mathrm{−0.50\: V}\]
A larger reduction potential means that it is easier to reduce the reactant. Permanganate, with the largest reduction potential, is the strongest oxidizer under these conditions. Dichromate is next, followed by titanium dioxide as the weakest oxidizing agent (the hardest to reduce) of this set.
Predict what reaction (if any) will occur between HCl and Co(s), and between HBr and Pt(s). You will need to use the standard reduction potentials from (Table P1).
Answer
\(\ce{Co}(s)+\ce{2HCl}⟶\ce{H2}+\ce{CoCl2}(aq)\); no reaction because Pt(s) will not be oxidized by H+
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
Prof. Robert J. Lancashire (The Department of Chemistry, University of the West Indies)

