Lavoisier
- Page ID
- 54109
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Understand why Lavoisier is sometimes called "the Father of Modern Chemistry"
- Distinguish accuracy from precision
So what happened to turn alchemy, which was like magical potion-brewing in Harry Potter, into the science of chemistry? It was measurement. Careful, careful measurement of quantities, such as masses, volumes, densities, temperatures, pressures.
An early hero of measurement was Antoine Lavoisier. He was one of the first true chemical scientists. He conducted careful experiments, and tried to draw no conclusions except those required by his data. He said fact, idea, and word should be as closely connected as possible: that you can't improve your language without improving your thinking, and you can't improve your thinking without improving your language. So he pioneered a systematic chemical nomenclature that is essentially what we use today. Remarkably, if you read his text, written in 1789, intended to introduce chemistry to beginners, much of it is still perfectly understandable and even correct by modern standards.
.jpg?revision=1&size=bestfit&width=200&height=272)
Lavoisier first describes the states of matter: gases, liquids and solids. He points out when a solid material is heated, it tends to expand, becoming first a liquid, which takes up a constant volume, but can be poured, unlike a solid. More heating, and it becomes a gas, which he describes as elastic because it will expand or compress to different volumes depending on the pressure. Unlike the Greek philosophers, he understood that this is a physical change, not a chemical change, and he has a good submicroscopic-scale intuition of what's happening: the particles of the material don't change, they just get further apart.
He recognized the following as elements: oxygen, nitrogen, hydrogen, sulfur, phosphorus, chlorine and fluorine (although he did not know their elemental forms), carbon, iron, copper, silver, gold, mercury, lead, tin, antimony, arsenic, bismuth, cobalt, manganese, molybdenum, nickel, platinum, tungsten, and zinc.
He burned sulfur and phosphorus and charcoal (carbon) and made careful observations, often using the bell jar over a bucket of mercury as shown in the drawing from his book, Figure 1. This is an example of a chemical change or chemical reaction, in which reactant chemicals turn into different product chemicals. If you light the sulfur in the dish labeled D under the bell jar of air, it burns until it goes out leaving some extra sulfur. The air remaining in the jar is no longer good for breathing. If you put a mouse in the jar, it will die, just as the flame did. This demonstrates the concept of limiting reactant. The reaction or burning stopped when it ran out of oxygen, leaving primarily nitrogen (and a few trace other gases) in the jar. Priestley, another scientist, showed him how to prepare pure oxygen gas, and he used this to do many burning experiments as well.
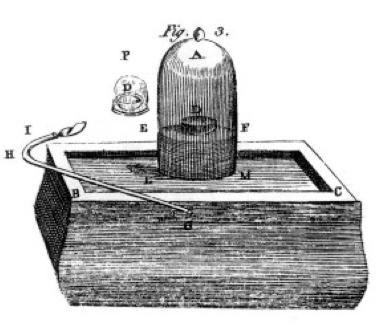
Lavoisier was obsessed with measurement. He developed elaborate apparatus for measuring everything. He would burn phosphorus, as shown in Figure 1, and observe the formation of a white flaky product. The phosphorus (the reactant in this case) wasn't water soluble, but the product was, so he collected the product very carefully, separating it from the unreacted phosphorus by washing with water. After drying, he could measure how much phosphorus had burned, how much oxygen had been consumed (because he knew the density of oxygen gas), and how much product had formed. He found that the mass of product was the sum of the masses of reactant consumed, in every experiment. This is the law of conservation of mass (which, actually, some earlier alchemists and chemists had also used). He also observed that the phosphorus has no taste, but the product, which he called phosphoric acid, is sour. He knew from these experiments that in many cases elements combine in only certain proportions, and also that oxygen can combine with sulfur, phosphorus, etc in two different ratios. He gave us the terminology we still use today: sulfuric acid is composed of sulfur and more oxygen, sulfurous acid is composed of sulfur and less oxygen. -ous means less oxygen; -ic means more oxygen. See the nomenclature page for details.
Lavoisier paid close attention to accuracy and precision. For instance, in the experiment we just described, he measured the volume of gas in the bell jar, before and after the reaction, but noted that after the reaction, you must wait until the temperature returns to what it was when you measured originally. If the gas is hot when you measure its volume after the reaction, it will have expanded, and your standard density will not apply. This would introduce a systematic error into the measurements: each time you perform the experiment, you will think that there is more gas leftover than there actually is, and your measurement won't be accurate. If the average result of your experiment is near the correct value, it is accurate. However, if your experiment gives very different numbers each time, even if the average is correct and the experiment is accurate, it is not precise. Precision is the difference between meeting "around 2 o'clock" and meeting "at 3 minutes and 27 seconds before 2 pm." Precision is how specific you are, how much detail you use. Lavoisier also helped develop the system of units (kg, L, m) that are currently in use in Korea and many other countries.

Overall, while he didn't do very many original experiments that nobody else had done before, he did his experiments very carefully, so they were as accurate and precise as possible, and then he thought about them clearly and created words to describe the chemicals and ideas that helped make everything clearer. If you read a chemistry textbook written before Lavoiser, you will be very confused because the names for chemicals would be based on history (and would sound like they came from Harry Potter), rather than being based on what the chemicals are. If you read a chemistry text written after Lavoiser, you will recognize the language as similar to what we use today.
Summary
Accuracy describes how close a measured value is to the actual value. Precision describes how well a group of measured values agree with each other. The law of conservation of mass states that matter can neither be created nor destroyed by a chemical or physical process. This results in the sum of the masses of reactant consumed in any experiment is equal to the mass of product. Chemical changes involve changing a substance's chemical identity such that new substances are formed. Physical changes involve altering a substance without changing its chemical identity. Combustion and rusting are two examples of chemical processes while boiling and melting are examples of physical processes. Chemical reactions involve turning reactants, chemicals that get consumed in the process of chemical change, into products, chemicals produced through the process of chemical change that have a different composition from the reactants. A limiting reactant determines, or limits, the amount of product that can be produced from a chemical reaction.
Outside Link
Contributors and Attributions
Emily V Eames (City College of San Francisco)

