11.10: Weak Acids
- Page ID
- 49504
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Not all molecules which contain hydrogen are capable of donating protons. For example, methane (CH4) and other hydrocarbons show no acidic properties at all. Carbon is not highly electronegative, and so electron density is fairly evenly shared in a C―H bond, and the hydrogen atom is unlikely to depart without at least one electron. Even when it is bonded to highly electronegative atoms like oxygen or fluorine, a hydrogen atom is not always strongly acidic.
Acetic acid has the projection formula
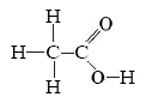
Write an equation for transfer of a proton from acetic acid to water.
Solution
By the electronegativity rule given above, only the hydrogen attached to oxygen should be acidic. The equation is
\[\text{CH}_{3}\text{COOH} + \text{H}_{2}\text{O} \rightleftharpoons \text{H}_{3}\text{O}^{+} + \text{CH}_{3}\text{COO}^{-} \label{1} \]
Note: To emphasize that only one hydrogen atom is acidic, the formula acetic acid is often written HC2H3O2. You may also find the formulas HAc or HOAc for acetic acid, where Ac– or OAc– represents the acetate ion, CH3COO–.
The equation for the proton transfer between acetic acid and water is written with a double arrow because it occurs to only a limited extent. Like HgCl2, acetic acid is a weak electrolyte. According to Table 1 in Ions in Solution (Electrolytes), 0.001 M HC2H3O2 conducts slightly more than one-tenth as much current as the same concentration of the strong acids HCl or HNO3. Therefore we can conclude that at a given instant only a little over 10 percent of the acetic acid molecules have donated protons to water molecules according to Eq. \(\ref{1}\). Nearly 90 percent are in molecular form as CH3COOH (or HC2H3O2) and make no contribution to the current. Because acetic acid is not a strong enough proton donor to be entirely converted to hydronium ions in aqueous solution, it is called a weak acid. A given concentration of a weak acid produces fewer hydronium ions per unit volume and therefore less acidity than the same concentration of a strong acid.
For those of you in laboratory, you can identify a weak acid using litmus paper. Below is an image of litmus paper after the addition of the weak acid acetic acid (CH3COOH). Notice the red hue, which indicates a weak acid. If HCl of the same molarity were tested, the color would be darker, more purple than red.
There are a large number of weak acids, but fortunately they fall into a few well-defined categories:
These compounds have the general formula RCOOH. All react with water in the same way as acetic acid [Eq. \(\ref{1}\)]. The strength of carboxylic acids is dependent on the electronegative strength of the atoms in the "R" group. Consider the compounds F3COOH and H3COOH. Fluorine is the most electronegative element, while hydrogen is comparable to carbon in electronegativity. Thus, the fluorines pull electron density away from the carboxyl group. This removes electron density from the acidic oxygen-hydrogen bond, which weakens it. This weaker bond means that the hydrogen can be removed more easily, which creates a stronger acid. This concept can be applied to any R group. The more electronegative the R group, the stronger the carboxylic acid will be.
Weak oxyacids
These have the same general formula HnXOm as strong oxyacids, but the number of hydrogens is equal to or one less than the number of oxygens. For a weak oxyacid, in other words, m ≤ n + 1. Some examples are:
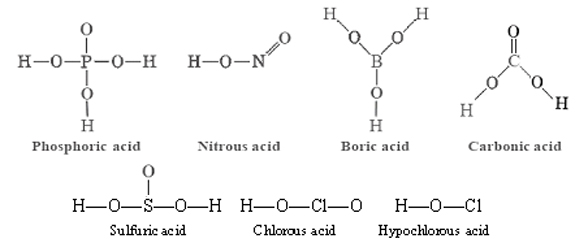
Some of the weak oxyacids, H2CO3 for example, are very unstable and cannot be separated in pure form from aqueous solution. Other molecules containing acidic hydrogen atoms
Hydrogen fluoride (HF) has a very strong bond and does not donate its proton as readily as other hydrogen halides. Other molecules in this category are hydrogen sulfide (H2S) and hydrogen cyanide (HCN). In the latter case, even though H is bonded to C, the electronegative N atom pulls some electron density away, and the HCN molecule is a very weak proton donor.
Hydrated cations
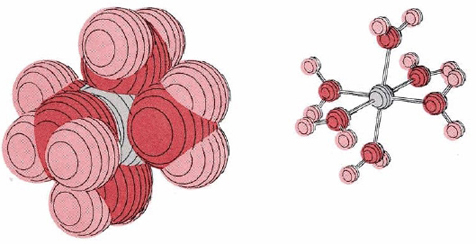
Cations, especially those of charge +3 or more or of the transition metals, are surrounded closely by four to six water molecules in aqueous solution. An example is Cr(H2O)63+, shown in Figure \(\PageIndex{1}\). The positive charge of the metal ion pulls electron density away from the surrounding water molecules, weakening the hold of the oxygen atoms for the hydrogen atoms. The latter can consequently be more easily donated as protons:
\[\text{Cr}(\text{H}_{2}\text{O})_{6}^{3+} + \text{H}_{2}\text{O} \rightleftharpoons \text{Cr}(\text{H}_{2}\text{O})_{5}\text{OH}^{2+} + \text{H}_{3}\text{O}^{+} \nonumber \]
Ions having acidic protons
Certain other ions can donate protons. One example is the ammonium ion, NH4+:
\[\text{NH}_{4}^{+} + \text{H}_{2}\text{O} \rightleftharpoons \text{NH}_{3} + \text{H}_{3}\text{O}^{+} \nonumber \]
Anions formed when some acids donate protons can lose yet another H+. An example of this is the hydrogen sulfate ion formed when sulfuric acid donates a proton:
\[\text{H}_{2}\text{SO}_{4} + \text{H}_{2}\text{O} \rightarrow \text{H}_{3}\text{O}^{+} + \text{HSO}_{4}^{-} \nonumber \]
\[\text{HSO}_{4}^{-} + \text{H}_{2}\text{O} \rightleftharpoons \text{H}_{3}\text{O}^{+} + \text{SO}_{4}^{2-} \nonumber \]
Although sulfuric acid is strong, the negative charge on the hydrogen sulfate ion holds the proton tighter, and so the ion is a considerably weaker acid. Acids such as H2SO4, H2S, H2SO3, and H2CO3 are called diprotic because they can donate two protons. Phosphoric acid, H3PO4, is triprotic—it can donate three protons.


