8.6: Reverse Osmosis
- Page ID
- 78411
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- What is reverse osmosis, and what is its principal application?
- Explain the role of osmotic pressure in food preservation, and give an example.
- Describe the role osmosis plays in the rise of water in plants (where is the semipermeable membrane?), and why it cannot be the only cause in very tall trees.
\[E=mc^2\]
If it takes a pressure of \(Π\) atm to bring about osmotic equilibrium, then it follows that applying a hydrostatic pressure greater than this to the high-solute side of an osmotic cell will force water to flow back into the fresh-water side. This process, known as reverse osmosis, is now the major technology employed to desalinate ocean water and to reclaim "used" water from power plants, runoff, and even from sewage. It is also widely used to deionize ordinary water and to purify it for for industrial uses (especially beverage and food manufacture) and drinking purposes.
![osmosis2[1].png](https://chem.libretexts.org/@api/deki/files/125159/osmosis2%255B1%255D.png?revision=1)
![revosmosis-1[1].png](https://chem.libretexts.org/@api/deki/files/125158/revosmosis-1%255B1%255D.png?revision=1)
Pre-treatment commonly employs activated-carbon filtration to remove organics and chlorine (which tends to damage RO membranes). Although bacteria are unable to pass through semipermeable membranes, the latter can develop pinhole leaks, so some form of disinfection is often advised. The efficiency and cost or RO is critically dependent on the properties of the semipermeable membrane.
![ro_tampa[1].jpg](https://chem.libretexts.org/@api/deki/files/125160/ro_tampa%255B1%255D.jpg?revision=1&size=bestfit&width=305&height=328)
![ro_home[1].jpg](https://chem.libretexts.org/@api/deki/files/125161/ro_home%255B1%255D.jpg?revision=1&size=bestfit&width=278&height=328)
Osmotic Generation of Electric Power
The osmotic pressure of seawater is almost 26 atm. Since a pressure of 1 atm will support a column of water 10.6 m high, this means that osmotic flow of fresh water through a semipermeable membrane into seawater could in principle support a column of the latter by 26 x 10.3 = 276 m (904 ft)!
So imagine an osmotic cell in which one side is supplied with fresh water from a river, and the other side with seawater. Osmotic flow of fresh water into the seawater side forces the latter up through a riser containing a turbine connected to a generator, thus providing a constant and fuel-less source of electricity. The key component of such a scheme, first proposed by an Israeli scientist in 1973 and known as pressure-retarded osmosis (PRO) is of course a semipermeable membrane capable of passing water at a sufficiently high rate.
![osmosis-power[1].png](https://chem.libretexts.org/@api/deki/files/125162/osmosis-power%255B1%255D.png?revision=1)
The world's first experimental PRO plant was opened in 2009 in Norway. Its capacity is only 4 kW, but it serves as proof-in-principle of a scheme that is estimated capable of supplying up to 2000 terawatt-hours of energy worldwide. The semipermeable membrane operates at a pressure of about 10 atm and passes 10 L of water per second, generating about 1 watt per m2 of membrane. PRO is but one form of salinity gradient power that depends on the difference between the salt concentrations in different bodies of water.
1 atm is equivalent to 1034 g cm–2, so from the density of water we get (1034 g cm–2) ÷ (1 g cm–3) = 1034 cm = 10.3 m.
Osmosis in Biology and Physiology
Because many plant and animal cell membranes and tissues tend to be permeable to water and other small molecules, osmotic flow plays an essential role in many physiological processes.
Normal saline solution
The interiors of cells contain salts and other solutes that dilute the intracellular water. If the cell membrane is permeable to water, placing the cell in contact with pure water will draw water into the cell, tending to rupture it. This is easily and dramatically seen if red blood cells are placed in a drop of water and observed through a microscope as they burst. This is the reason that "normal saline solution", rather than pure water, is administered in order to maintain blood volume or to infuse therapeutic agents during medical procedures.
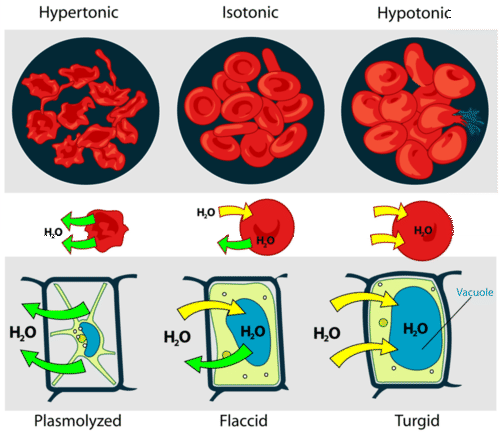
In order to prevent irritation of sensitive membranes, one should always add some salt to water used to irrigate the eyes, nose, throat or bowel. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 M, making its osmotic pressure close to that of blood.
Food preservation
The drying of fruit, the use of sugar to preserve jams and jellies, and the use of salt to preserve certain meats, are age-old methods of preserving food. The idea is to reduce the water concentration to a level below that in living organisms. Any bacterial cell that wanders into such a medium will have water osmotically drawn out of it, and will die of dehydration. A similar effect is noticed by anyone who holds a hard sugar candy against the inner wall of the mouth for an extended time; the affected surface becomes dehydrated and noticeably rough when touched by the tongue.
In the food industry, what is known as water activity is measured on a scale of 0 to 1, where 0 indicates no water and 1 indicates all water. Food spoilage micro-organisms, in general, are inhibited in food where the water activity is below 0.6. However, if the pH of the food is less than 4.6, micro-organisms are inhibited (but not immediately killed] when the water activity is below 0.85.
Diarrhea
The presence of excessive solutes in the bowel draws water from the intestinal walls, giving rise to diarrhea. This can occur when a food is eaten that cannot be properly digested (as, for example, milk in lactose-intolerant people). The undigested material contributes to the solute concentration, raising its osmotic pressure. The situation is made even worse if the material undergoes bacterial fermentation which results in the formation of methane and carbon dioxide, producing a frothy discharge.
Water Transport in Plants
Osmotic flow plays an important role in the transport of water from its source in the soil to its release by transpiration from the leaves, it is helped along by hydrogen-bonding forces between the water molecules. Capillary rise is not believed to be a significant factor.
Water enters the roots via osmosis, driven by the low water concentration inside the roots that is maintained by both the active [non-osmotic] transport of ionic nutrients from the soil and by the supply of sugars that are photosynthesized in the leaves. This generates a certain amount of root pressure which sends the water molecules on their way up through the vascular channels of the stem or trunk. But the maximum root pressures that have been measured can push water up only about 20 meters, whereas the tallest trees exceed 100 meters. Root pressure can be the sole driver of water transport in short plants, or even in tall ones such as trees that are not in leaf. Anyone who has seen apparently tender and fragile plants pushing their way up through asphalt pavement cannot help but be impressed!
But when taller plants are actively transpiring (losing water to the atmosphere], osmosis gets a boost from what plant physiologists call cohesion tension or transpirational pull. As each H2O molecule emerges from the opening in the leaf it pulls along the chain of molecules beneath it. So hydrogen-bonding is no less important than osmosis in the overall water transport process. If the soil becomes dry or saline, the osmotic pressure outside the root becomes greater than that inside the plant, and the plant suffers from “water tension”, i.e., wilting.
Do fish drink water? Do they Urinate?
The following section is a bit long, but for those who are interested in biology it offers a beautiful example of how the constraints imposed by osmosis have guided the evolution of ocean-living creatures into fresh-water species . It concerns ammonia NH3, a product of protein metabolism that is generated within all animals, but is highly toxic and must be eliminated.
Marine invertebrates (those that live in seawater) are covered in membranes that are fairly permeable to water and to small molecules such as ammonia. So water can diffuse in either direction as required, and ammonia can diffuse out as quickly as it forms. Nothing special here.
Invertebrates that live in fresh water do have problem: the salt concentrations within their bodies are around 1%, much greater than in fresh water. For this reason they have evolved surrounding membranes that are largely impermeable to salts (to prevent their diffusion out of the body) and to water (to prevent osmotic flow in.) But these organisms must also be able to exchange oxygen and carbon dioxide with their environment. The special respiratory organs (gills) that mediate this process, as a consequence of being permeable to these two gases, will also allow water molecules (whose sizes are comparable to those of the respiratory gases) to pass through. In order to protect fresh-water invertebrates from the disastrous effects of unlimited water inflow through the gill membranes, these animals possess special excretory organs that expel excess water back into the environment. Thus in such animals, there is a constant flow of water passing through the body. Ammonia and other substances that need to be excreted are taken up by this stream which constitutes a continual flow of dilute urine.
Fishes fall into two general classes: most fish have bony skeletons and are known as teleosts. Sharks and rays have cartilage instead of bones, and are called elasmobranchs. For the teleosts that live in fresh water, the situation is very much the same as with fresh-water invertebrates; they take in and excrete water continuously. The fact that an animal lives in the water does not mean that it enjoys an unlimited supply of water. Marine teleosts have a more difficult problem. Their gills are permeable to water, as are those of marine invertebrates. But the salt content of seawater (about 3%), being higher than the about 1% in the fish’s blood, would draw water out of the fish. Thus these animals are constantly losing water, and would be liable to desiccation if water could freely pass out of their gills. Some does, of course, and with it goes most of its nitrogen in the form of NH3.
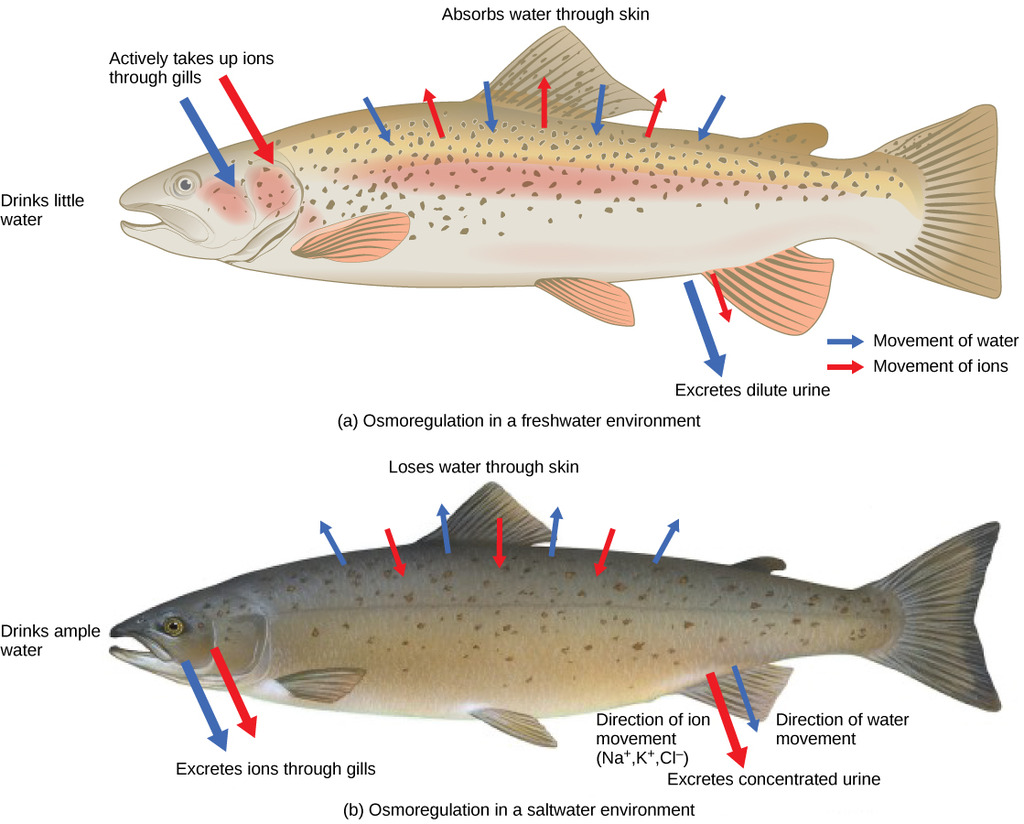
Thus most of the waste nitrogen exits not through the usual excretory organs as with most vertebrates, but through the gills. But in order to prevent excessive loss of water, the gills have reduced permeability to this water, and with it, to comparably-sized NH3. So in order to prevent ammonia toxicity, the remainder of it is converted to a non-toxic substance (trimethylamine oxide (CH3)3NO) which is excreted via the kidneys.
The marine elasmobranchs solve the loss-of-water problem in another way: they convert waste ammonia to urea (NH3)2CO which is highly soluble and non-toxic. Their kidneys are able to control the quantity of urea excreted so that their blood retains about 2-2.5 percent of this substance. Combined with the 1 percent of salts and other substances in their blood, this raises the osmotic pressure within the animal to slightly above that of seawater, Thus the same mechanism that protects them from ammonia poisoning also ensures them an adequate water supply.


