7.2: Acid-Base Reactions: A Guide for Beginners
- Page ID
- 357189
Let us begin with the hydrogen chloride and water reaction from the last chapter, a classic acid–base reaction. To understand how these types of reactions are related, we need to learn how to identify their essential and common components. Our first hurdle is the fact that the terms acid and acidity, and to a lesser extent, bases and basicity, have entered the language of everyday life. Most people have some notion of acids and acidity. Examples of common usage include: acid rain, stomach acid, acid reflux, acid tongue, etc. You might hear someone talk about wine that tastes acidic, by which they probably mean sour, and most people would nod their heads in comprehension. Old wine tastes like vinegar because it contains acetic acid. You have also probably heard of or even learned about measurements of acidity that involve \(\mathrm{pH}\), but what is \(\mathrm{pH}\) exactly? What is an acid, and why would you want to neutralize it? Are acidic things bad? Do we need to avoid them at all costs and under all circumstances? Although the term base is less common, you may already be familiar with materials that are basic in the chemical sense. Bases are often called alkalis, as in alkaline batteries and alkali metals. They are slippery to the touch, bitter tasting.
Not surprisingly, many definitions of acid–base reactions have been developed over the years. Each new definition has been consistent, that is it produces similar conclusions when applied to a particular system, to the ones that have come before, but each new definition has also furthered the evolution of the idea of acids and bases. Later definitions encompass original ideas about acids and bases, but also broaden them and make them more widely applicable, covering a large array of reactions with similar characteristics. We will start with the simplest model of acids and bases—the Arrhenius model.[3] This is the most common introduction to acid–base chemistry; perhaps you have already been taught this model. Although the Arrhenius model is of limited usefulness, we will examine its simple structure as the foundation for more sophisticated and useful models. Our model-by-model consideration should help you appreciate how acid–base chemistry has become increasingly general, and powerful over time. As we progress, keep this simple rule in mind: All acid–base reactions begin and end with polarized molecules. As we go through the various models for acid–base reactions, see if you can identify the polar groups and how they interact with each other.
Arrhenius Acids and Bases
In the Arrhenius model, an acid is defined as a compound that dissociates when dissolved in water to produce a proton (\(\mathrm{H}^{+}\)) and a negatively-charged ion (an anion). In fact, naked protons (\(\mathrm{H}^{+}\)) do not roam around in solution. They always associate with at least one, and more likely multiple, water molecules. [4] Generally, chemists use a shorthand for this situation, either referring to the \(\mathrm{H}^{+}\) in aqueous solution as a hydronium ion (denoted as \(\mathrm{H}_{3}\mathrm{O}^{+}\)) or even more simply as \(\mathrm{H}^{+}\), but do not forget, this is a short-hand. An example of an Arrhenius acid reaction is: \[\mathrm{HCl}(g)+\mathrm{H}_{2} \mathrm{O} \rightleftarrows \mathrm{H}_{3} \mathrm{O}^{+}(aq)+\mathrm{Cl}^{-}(aq)\]
or, more simply (and truer to the original theory): \[\mathrm{HCl}(g) \rightleftarrows \mathrm{H}^{+}(aq)+\mathrm{Cl}^{-}(aq) \text { or } \mathrm{HCl}(aq)\]
But this is really quite a weird way to present the actual situation, because the \(\mathrm{HCl}\) molecule does not interact with a single water molecule, but rather interacts with water as a solvent. When hydrogen chloride (\(\mathrm{HCl}\)) gas is dissolved in water, it dissociates into \(\mathrm{H}^{+}(aq)\) and \(\mathrm{Cl}^{-}(aq)\) almost completely. For all intents and purposes, there are no \(\mathrm{HCl}\) molecules in the solution. An aqueous solution of \(\mathrm{HCl}\) is known as hydrochloric acid, which distinguishes it from the gas, hydrogen chloride. This complete dissociation is a characteristic of strong acids, but not all acids are strong!
An Arrhenius base is defined as a compound that generates hydroxide (\(–\mathrm{OH}\)) ions when dissolved in water. The most common examples of Arrhenius bases are the Group I (alkali metal) hydroxides, such as sodium hydroxide: \[\mathrm{NaOH}(s)+\mathrm{H}_{2} \mathrm{O} \rightleftarrows \mathrm{Na}^{+}(aq)+{ }^{-} \mathrm{OH}(aq) \text { or } \mathrm{NaOH}(aq)\]
Again, this is a reaction system that involves both \(\mathrm{NaOH}\) and liquid water. The process of forming a solution of sodium hydroxide is just like the one involved in the interaction between sodium chloride (\(\mathrm{NaCl}\)) and water: the ions (\(\mathrm{Na}^{+}\) and \({}^{-}\mathrm{OH}\)) separate and are solvated (surrounded) by the water molecules.
As we will see shortly, some acids (and bases) do not ionize completely; some of the acid molecules remain intact when they dissolve in water. When this occurs we use double-headed arrows \(\rightleftarrows\) to indicate that the reaction is reversible, and both reactants and products are present in the same reaction mixture. We will have much more to say about the duration and direction of a reaction in the next chapter. For now, it is enough to understand that acid–base reactions (in fact, all reactions) are reversible at the molecular level. In the case of simple Arrhenius acids and bases, however, we can assume that the reaction proceeds almost exclusively to the right.
An Arrhenius acid–base reaction occurs when a dissolved (aqueous) acid and a dissolved (aqueous) base are mixed together. The product of such a reaction is usually said to be a salt plus water and the reaction is often called a neutralization reaction: the acid neutralizes the base, and vice versa. The equation can be written like this: \[\mathrm{HCl}(aq)+\mathrm{NaOH}(aq) \rightleftarrows \mathrm{H}_{2} \mathrm{O}(l)+\mathrm{NaCl}(aq)\]
When the reaction is written in this molecular form it is quite difficult to see what is actually happening. If we rewrite the equation to show all of the species involved, and assume that the number of \(\mathrm{HCl}\) and \(\mathrm{NaOH}\) molecules are equal, we get: \[\mathrm{H}^{+}(aq)+\mathrm{Cl}^{-}(aq)+\mathrm{Na}^{+}(aq)+-\mathrm{OH}(aq) \rightleftarrows \mathrm{H}_{2} \mathrm{O}(l)+\mathrm{Na}^{+}(aq)+\mathrm{Cl}^{-}(aq)\]
\(\mathrm{Na}^{+}(aq)\) and \(\mathrm{Cl}^{-}(aq)\) appear on both sides of the equation; they are unchanged and do not react (they are often called spectator ions because they do not participate in the reaction). The only actual reaction that occurs is the formation of water: \[\mathrm{H}^{+}(aq)+{ }^{-} \mathrm{OH}(aq) \rightleftarrows \mathrm{H}_{2} \mathrm{O}(l)\]
The formation of water (not the formation of a salt) is the signature of an Arrhenius acid–base reaction. A number of common strong acids, including hydrochloric acid (\(\mathrm{HCl}\)), sulfuric acid (\(\mathrm{H}_{2}\mathrm{SO}_{2}\)), and nitric acid (\(\mathrm{HNO}_{2}\)), react with a strong base such as \(\mathrm{NaOH}\) or \(\mathrm{KOH}\) (which, like strong acids, dissociate completely in water) to produce water..
Such acid–base reactions are always exothermic and we can measure the temperature change and calculate the corresponding enthalpy change (\(\Delta \mathrm{H}\)) for the reaction. Regardless of which strong acid or strong base you choose, the enthalpy change is always the same (about \(58 \mathrm{~kJ/mol}\) of \(\mathrm{H}_{2}\mathrm{O}\) produced). This is because the only consistent net reaction that takes place in a solution of a strong acid and a strong base is: \[\mathrm{H}^{+}(aq)+-\mathrm{OH}(aq) \rightleftarrows \mathrm{H}_{2} \mathrm{O}(l)\]
One other factor to note is that the overall reaction involves a new bond being formed between the proton (\(\mathrm{H}^{+}\)) and the oxygen of the hydroxide (\({}^{-}\mathrm{OH}\).) It makes sense that something with a positive charge would be attracted to (and bond with) a negatively-charged species (although you should recall why the \(\mathrm{Na}^{+}\) and \(\mathrm{Cl}^{-}\) do not combine to form sodium chloride solid in aqueous solution.) Whether or not bonds form depends on the exact nature of the system, and the enthalpy and entropy changes that are associated with the change. We will return to this idea later in chapter \(8\).
Questions to Answer
- What would be the reaction if equal amounts of equimolar \(\mathrm{HNO}_{3}\) and \(\mathrm{KOH}\) were mixed?
- How about equal amounts of equimolar \(\mathrm{H}_{2}\mathrm{SO}_{4}\) and \(\mathrm{KOH}\)? What would the products be?
- How about equal amounts of equimolar \(\mathrm{H}_{3}\mathrm{PO}_{4}\) and \(\mathrm{KOH}\)?
- How many moles of \(\mathrm{NaOH}\) would be needed to react fully with one mole of \(\mathrm{H}_{3}\mathrm{PO}_{4}\)?
- Draw a molecular level picture of Arrhenius acid base reaction.
Brønsted–Lowry[5] Acids and Bases
The Arrhenius acid–base model is fairly easy to understand but its application is limited to certain kinds of reactions. Rather than continue down this road, chemists found that they needed to expand their model of acids and bases and how they react. The first of these expansions was the Brønsted–Lowry model. In the Brønsted–Lowry model, an acid is characterized as a proton (\(\mathrm{H}^{+}\)) donor and a base as a proton acceptor. If we revisit the reactions we looked at earlier in the context of the Brønsted–Lowry acid-base model, we see that \(\mathrm{HCl}\) is the proton donor; it gives away \(\mathrm{H}^{+}\) and water is the proton acceptor. In this scheme, \(\mathrm{HCl}\) is the acid and water is the base: \[\mathrm{HCl}(g)+ \mathrm{H}_{2} \mathrm{O}(l) \rightleftarrows \mathrm{H}_{3} \mathrm{O}^{+}(aq)+\mathrm{Cl}^{-}(aq)\]
\(\mathrm{HCl} = \) acid \(\mathrm{H}_{2} \mathrm{O} = \) base \(\mathrm{H}_{3} \mathrm{O}^{+} = \) conjugate acid \(\mathrm{Cl}^{-} = \) conjugate base
The resulting species are called the conjugate acid (so \(\mathrm{H}_{3} \mathrm{O}^{+}\) is the conjugate acid of \(\mathrm{H}_{2} \mathrm{O}\) and the conjugate base (\(\mathrm{Cl}^{-}\) is the conjugate base of \(\mathrm{HCl}\)). This is because \(\mathrm{H}_{3} \mathrm{O}^{+}\) can and generally does donate its \(\mathrm{H}^{+}\) to another molecule (most often another water molecule) and \(\mathrm{Cl}^{-}\) can accept an \(\mathrm{H}^{+}\).
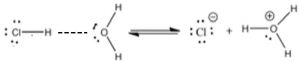
A major (and important difference) between the Brønsted–Lowry and Arrhenius acid–base models is that a Brønsted–Lowry acid must always have an accompanying base to react with—the two are inseparable. A proton donor must have something to donate the protons to (a base)—in this case, water. Remember that bond breaking requires energy, whereas bond formation releases energy. Some energy input is always required for a reaction in which the only thing that happens is the breaking of a bond (for example the \(\mathrm{Cl–H}\) bond in \(\mathrm{HCl}\)). Acid–base reactions are typically exothermic; they release energy to the surroundings and the released energy is associated with the interaction between the \(\mathrm{H}^{+}\) and the base. In other words, the proton does not drop off the acid and then bond with the base. Instead, the acid\(\mathrm{–H}\) bond starts to break as the base–H bond starts to form. One way that we can visualize this process is to draw out the Lewis structures of the molecules involved and see how the proton is transferred.
As shown in the figure, we use a dotted line to show the growing attraction between the partial positive charge on the \(\mathrm{H}\) of the \(\mathrm{H—Cl}\) molecule and the partial negative charge on the oxygen. This interaction results in the destabilization of the \(\mathrm{H—Cl}\) bond. Because the \(\mathrm{Cl}\) is more electronegative than the \(\mathrm{H}\), the electrons of the original \(\mathrm{H—Cl}\) bond remain with the \(\mathrm{Cl}\) (which becomes \(\mathrm{Cl}^{-}\)) and the \(\mathrm{H}^{+}\) forms a new bond with a water molecule. Essentially, a Brønsted–Lowry acid–base reaction involves the transfer of a proton from an acid to a base, leaving behind the original bonding electrons.
Another example of an acid–base reaction is the reaction of ammonia with water: \[\mathrm{NH}_{3}(aq)+\mathrm{H}_{2} \mathrm{O}(l) \rightleftarrows \mathrm{NH}_{4}^{+}(aq)+{}^{-} \mathrm{OH}(aq)\]
\(\mathrm{NH}_{3} = \) base \(\mathrm{H}_{2} \mathrm{O} = \) acid \(\mathrm{NH}_{4} = \) conjugate acid \({}^{-} \mathrm{OH} = \) conjugate base

In this case, oxygen is more electronegative than nitrogen. The proton is transferred from the oxygen to the nitrogen. Again, the dotted line in the figure represents the developing bond between the hydrogen and the nitrogen. As the \(\mathrm{H—O}\) bond breaks, a new \(\mathrm{H—N}\) bond forms, making the resulting \(\mathrm{NH}_{4} {}^{+}\) molecule positively-charged. The electrons associated with the original \(\mathrm{H—O}\) bond are retained by the \(\mathrm{O}\), making it negatively-charged. So, water is the acid and ammonia is the base! An important difference between this and the preceding \(\mathrm{HCl–H}_{2}\mathrm{O}\) reaction is that \(\mathrm{H}_{2}\mathrm{O}\) is a much weaker acid than is \(\mathrm{HCl}\). In aqueous solution, not all of the \(\mathrm{NH}_{3}\) reacts with \(\mathrm{H}_{2}\mathrm{O}\) to form \(\mathrm{NH}_{4} {}^{+}\). Moreover, the reaction between \(\mathrm{NH}_{3}\) and water is reversible, as indicated by the \(\rightleftarrows\) symbol. The next chapter will consider the extent to which a reaction proceeds to completion. You may be wondering why the water does not act as a base in the reaction with \(\mathrm{NH}_{3}\), like it does with \(\mathrm{HCl}\). If you draw out the products resulting from a proton transfer from nitrogen to oxygen, you will see that this process results in a mixture of products where the more electronegative atom (\(\mathrm{O}\)) now has a positive charge, and the less electronegative atom (\(\mathrm{N}\)) has a negative charge. It does not make sense that the most electronegative atom would end up with a positive charge, and indeed this process does not happen (to any measurable extent).
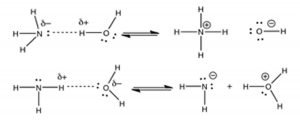
We will soon return to a discussion of what makes a compound acidic and/or basic. At the moment, we have two acid–base reactions: one in which water is the acid and the other in which water is the base. How can this be? How can one molecule of water be both an acid and a base, apparently at the same time? It is possible because of the water molecule’s unique structure. In fact, water reacts with itself, with one molecule acting as an acid and one as a base: \[\mathrm{H}_{2} \mathrm{O}(l)+\mathrm{H}_{2} \mathrm{O}(l) \rightleftarrows \mathrm{H}_{3} \mathrm{O}^{+}(aq)+{ }^{-} \mathrm{OH}(aq)\]
\(\mathrm{H}_{2} \mathrm{O} = \) acid \(\mathrm{H}_{2} \mathrm{O} = \) base \(\mathrm{H}_{3} \mathrm{O}^{+} = \) conjugate acid \({}^{-} \mathrm{OH} = \) conjugate base
As shown in the figure, we can again visualize this process by drawing out the Lewis structures of the water molecules to see how the proton is able to move from one water molecule to another, so that it is never “alone” and always interacting with the lone pairs on the oxygens.
Questions to Ponder
- Between the Arrhenius model and the Brønsted–Lowry model of acids and base, which is more useful? Why?
Questions to Answer
- Which do you think is more likely to happen? The reaction \(\mathrm{H}_{2} \mathrm{O} + \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{H}_{3} \mathrm{O}^{+} + { }^{-} \mathrm{OH}\)? Or the reverse process \(\mathrm{H}_{3} \mathrm{O}^{+} + { }^{-} \mathrm{OH} \rightarrow \mathrm{H}_{2} \mathrm{O} + \mathrm{H}_{2} \mathrm{O}\)? Could they both happen at once?
- What do you think the relative amounts of \(\mathrm{H}_{2} \mathrm{O}\), \(\mathrm{H}_{3} \mathrm{O}^{+} + { }^{-} \mathrm{OH}\) might be in a pure sample of liquid water? How would you measure the relative amounts?
- Now that you know \(\mathrm{HCl}\) is an acid and ammonia is a base, can you predict the reaction that occurs between them?
- Is water a necessary component of a Brønsted–Lowry acid–base reaction? How about for an Arrhenius acid–base reaction?
How to Spot an Acid
Moving on from water, can we predict whether a compound will be an acid, a base, or neither? We have learned that we can predict many properties of materials by considering their molecular structure. When acids are written in their simplified form (for example \(\mathrm{HNO}_{3}\) or \(\mathrm{H}_{2}\mathrm{SO}_{4}\)) it can be very difficult to see any similarities, but if we draw out the Lewis structures some commonalities emerge. Let us take a look at the Lewis structures for several strong acids, such as hydrochloric acid \(\mathrm{HCl}(aq)\), nitric acid \(\mathrm{HNO}_{3}(aq)\), and sulfuric acid \(\mathrm{H}_{2}\mathrm{SO}_{4}(aq)\).[6] What structural feature do these substances have in common? Well, from their formulae it is clear that they all contain hydrogen, but there are many compounds that contain hydrogen that are not acidic. For example, methane (\(\mathrm{CH}_{4}\)) and other hydrocarbons are not acidic; they do not donate protons to other molecules.
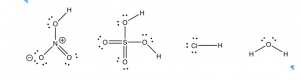
One common feature of acids is that the proton that gets donated (or picked off) is bonded to a highly electronegative atom. This atom is often either an oxygen or a halogen such as chlorine (\(\mathrm{Cl}\)), bromine (\(\mathrm{Br}\)), or iodine (\(\mathrm{I}\)). Once you know what to look for, it is quite easy to spot the potentially acidic sites in a molecule. For example, in the previous figure, you could circle the “vulnerable” hydrogens. The ability to spot donatable hydrogens is a useful skill that allows you to predict properties of more complex molecules. But why is a hydrogen that is covalently bonded to an electronegative element potentially acidic and donatable?
First, let us consider the \(\mathrm{O—H}\) bond. Based on our discussion of water molecules, we can predict that it is polarized, with a partial positive charge on the H and a partial negative on the \(\mathrm{O}\). In water, the \(\mathrm{H}\) is (on average) also part of a hydrogen bonding interaction with the oxygen of another water molecule. It turns out that it does not take much energy to break the original \(\mathrm{O—H}\) bond. Remember that \(\mathrm{H}^{+}\) does not just “drop off” the acid, but at the same time forms a bond with the base molecule. In fact, strong acid–base reactions are typically exothermic, meaning that the new bond formed between the proton (\(\mathrm{H}^{+}\)) and the base is stronger than the bond that was broken to release the \(\mathrm{H}^{+}\). The released energy raises the temperature of the surroundings. In an aqueous solution of a strong acid, hydrogen ions are moving rapidly and randomly from one oxygen to another. The energy for all this bond-breaking comes from the thermal motion of water molecules.
We must also consider what happens to the oxygen that gets left behind. When the acidic hydrogen is transferred, it leaves behind the electrons that were in the bond, giving that atom more electrons than it started with. The species left behind must be stable even with those extra electrons (the negative charge). In the example below, chloride ion \(\mathrm{Cl}^{-}(aq)\) is left behind when the proton gets transferred away. We know chloride is stable and common. It is not surprising that it is one of the products of the reaction. \[\mathrm{HCl}(g)+\mathrm{H}_{2} \mathrm{O}(l) \rightleftarrows \mathrm{H}_{3} \mathrm{O}^{+}(aq) +\mathrm{Cl}^{-}(aq)\]
\(\mathrm{HCl} = \) acid \(\mathrm{H}_{2} \mathrm{O} = \) base \(\mathrm{H}_{3} \mathrm{O}^{+} = \) conjugate acid \(\mathrm{Cl}^{-} = \) conjugate base
If you recall, electronegativity is a measure of the ability to attract (and retain) electrons.[7] Therefore, it makes sense that a negatively-charged, electronegative atom (like chlorine or oxygen) will be more stable than a negatively-charged, less electronegative atom (like carbon).
Questions to Answer
- What other atoms besides chlorine or oxygen are electronegative enough to stabilize those extra electrons?
- Draw the reactions of each of the strong acids with water: (\(\mathrm{HCl} (aq)\)), nitric acid (\(\mathrm{HNO}_{3} (aq)\)), sulfuric acid (\(\mathrm{H}_{2}\mathrm{SO}_{4} (aq)\)), hydrogen bromide (\(\mathrm{HBr} (aq)\)), and hydrogen iodide (\(\mathrm{HI} (aq)\)). What are the commonalities? What are the differences?
- Draw the structures of methanol (\(\mathrm{CH}_{3}\mathrm{OH}\)), acetic acid (\(\mathrm{CH}_{3}\mathrm{COOH}\)), and methane (\(\mathrm{CH}_{4}\)) and write a potential reaction with water. Label the conjugate acid–base pairs.
- Which reactions do you think are likely to occur? Why?
Questions for Later
- What other methods (besides having a strongly electronegative atom) might be available to stabilize the electrons (recall that one model of bonding allows for molecular orbitals that extend over more than two atoms)? We will return to this idea later.
Strong, Weak, Concentrated, and Dilute Acids and Bases
It can be very confusing when words have a different meaning in the scientific context than they do in everyday life. The words we use to describe solutions of acids and bases fall into this category of easily mixed-up definitions. We use the term strong to refer to acids that ionize completely in water, and weak for those acids that are only partially ionized (see Chapter \(8\) for more information on why). Strong and weak are used to describe an intrinsic property of the acid or base. The terms dilute and concentrated are used to describe the concentration of the acid in water. We could have a dilute solution (say \(0.1 \mathrm{~M}\)) of the strong acid hydrochloric acid, or a concentrated solution (say \(10 \mathrm{~M}\)) of the weak acid acetic acid. By contrast, when we refer to strong versus weak liquids in the everyday sense, we are referring to the concentration of the solution. For example, if you say, “This tea is very weak” or “I like my coffee strong” what you are really saying that you like a lot of tea or coffee dissolved in the solution you are drinking. It is important to remember this difference and understand that the scientific context can change the meaning of familiar words.
Questions to Answer
- Draw out molecular-level pictures of a dilute solution of a strong acid and a weak acid.
- Draw out molecular-level pictures of a concentrated solution of a strong acid and a weak acid.
- What are the similarities and differences between all the representations you have drawn?
- Consider what you have learned about the energy changes associated with the reaction of a strong acid with water. From a safety point of view, which of the following actions makes more sense when diluting a concentrated solution of a strong acid with water? Why?
- A. Add water slowly (dropwise) to the concentrated strong acid or
- B. Add the concentrated strong acid dropwise to water
Factors That Affect Acid Strength
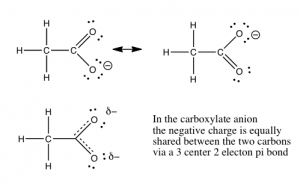
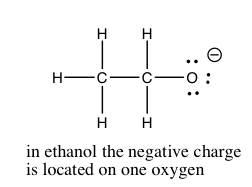
In Chapter \(8\), we will discuss the quantification of acid and base strength. First let us take a look at the factors that might affect the strength of an acid. As we have already seen, the ability of the conjugate base to hold on to (stabilize) the electron pair is crucial. There are several ways to accomplish this. The simplest is that the acidic \(\mathrm{H}\) is attached to an electronegative atom such as \(\mathrm{O}\), \(\mathrm{N}\), or a halogen. There is a wide range of acidities for oxyacids. The differences in acidity are determined by the number of places available for the extra electron density to be stabilized. The figure illustrates a fairly simple example of this in the difference between ethanol (\(\mathrm{CH}_{3}\mathrm{CH}_{2}\mathrm{OH}\)) and acetic acid (\(\mathrm{CH}_{3}\mathrm{COOH}\)). Acetic acid is about 10 billion times more acidic than ethanol, because the conjugate base (acetate) is able to stabilize the negative charge on two oxygens instead of just one. This “spreading out” of the charge diminishes the electron-electron repulsions, and stabilizes the structure more than if the negative charge were localized on just one oxygen. If you draw out the Lewis structures of the common strong inorganic oxy-acids (e.g. \(\mathrm{HNO}_{3}\) or \(\mathrm{H}_{2}\mathrm{SO}_{4}\)), you will see that it is possible to delocalize the negative charge of the corresponding anion on more than one oxygen.
How to Spot a Base
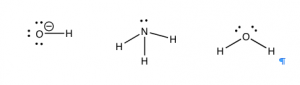
There is an equally simple method for figuring out which compounds are potential bases. Let us take a look at some common bases. The first bases that most people encounter are the metal hydroxides such as \(\mathrm{NaOH}\), \(\mathrm{KOH}\), and \(\mathrm{Mg}(\mathrm{OH})_{2}\). The metal ions are generated when these compounds dissolve in water, but they typically do not play any role in acid–base reactions.[8] The base in these compounds is the hydroxide (\({}^{-}\mathrm{OH}\)). Another common class of bases is molecules that contain nitrogen, like \(\mathrm{NH}_{3}\). There many kinds of nitrogenous bases, some of which play a critical role in biological systems. For example, the bases in nucleic acids (\(\mathrm{DNA}\) and \(\mathrm{RNA}\)) are basic because they contain nitrogen. Let us not forget that water is also basic and can accept a proton.
So what is the common structural feature in bases? Well, if an acid is the species with a proton to donate, then the base must be able to accept a proton. This means that the base must have somewhere for the proton to attach—it must contain a non-bonded (lone) pair of electrons for the proton to interact and bond with. If we look at our examples so far, we find that all the bases have the necessary non-bonded pair of electrons. Most common bases have either an oxygen or a nitrogen (with lone pairs of electrons) acting as the basic center. Once you learn how to spot the basic center, you can predict the outcome of a vast range of reactions rather than just memorizing them. It is often the case that if you can identify the acidic and basic sites in the starting materials, you can predict the product and ignore the rest of the molecule.
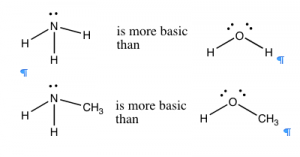
In general, nitrogen is a better proton acceptor than oxygen, because it is more basic. Ammonia (\(\mathrm{NH}_{3}\)) is more basic than water (\(\mathrm{H}_{2}\mathrm{O}\)), and organic compounds with nitrogen in them are typically more basic than the corresponding compounds containing structurally-analogous oxygens (\(\rightarrow\)). If we compare the trend in basicity for a range of simple compounds across the periodic table, we see that basicity decreases from \(\mathrm{NH}_{3} > \mathrm{~H}_{2}\mathrm{O} > \mathrm{~HF}\). This effect parallels the increase in electronegativity across the row. The ability to of an electron pair to bond with and accept a proton depends on how tightly that electron pair is held in by the donor atom. In fluorine, the most electronegative atom, the electrons are held so tightly and so close to the atom’s nucleus that they are not available to bond with a proton. Oxygen holds onto its electron pairs a little less tightly, and so is more likely than fluorine to donate a lone pair to a proton. Nitrogen, however, is even less electronegative and therefore has a more available lone pair, making most nitrogen compounds basic.[9]
Questions to Answer
- Why did we not include \(\mathrm{CH}_{4}\) or neon in this analysis?
- Do you think compounds with ammonium (\(\mathrm{NH}_{4} {}^{+}\)) are basic? Why or why not?
- Can you draw the structure of a basic compound that has not yet been mentioned in the text?
- Draw out the reactions of \(\mathrm{CH}_{3}\mathrm{NH}_{2}\) and \(\mathrm{CH}_{3}\mathrm{OH}\) with water. Label the conjugate acid and base pairs. Which reaction is most likely to occur? Why?
- How would you design an experiment to figure out whether a compound is an acid or a base (or both)? What experimental evidence would you accept to determine if you had an acid or a base or both?


