Solubility and Precipitation
- Page ID
- 53170
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Describe what occurs in a precipitation reaction
Precipitation is the process of a compound coming out of solution. It is the opposite of dissolution or solvation. In dissolution, the solute particles separate from each other and are surrounded by solvent molecules. In precipitation, the solute particles find each other and form a solid together. This solid is called the precipitate or sometimes abbreviated "ppt".
Solubility Equilibria
Precipitation and dissolution are a great example of a dynamic equilibrium (also described here). Any time there is a solution with a little bit of solid solute in it, both processes will be happening at once. Some molecules or ions will leave the solid and become solvated, and some solvated solute particles will bump into the solid and get stuck there. The rates of the 2 processes determine the overall effect: if precipitation happens faster, then a lot of solid can come out of the solution very quickly. If dissolution happens faster, than the solid will dissolve. As the solution becomes more concentrated, the rate of precipitation will increase and the rate of dissolution will decrease, so that eventually the concentration will stop changing, and this is equilibrium. When equilibrium is reached, the solution is saturated, and that concentration defines the solubility of the solute. Solubility is the maximum possible concentration, and it is given in M, g/L, or other units. Solubility changes with temperature, so if you look up solubility data it will specify the temperature.
Precipitation Reactions
Precipitation can happen for various reasons, such as that you cooled a solution, or removed some solvent by evaporation, or both. (This is often used as a way to purify a compound.) You can also have a precipitation reaction, when you mix two solutions together and a new combination of ions is super-saturated in the combined solution. For example, maybe you mixed a solution of silver(I) nitrate and sodium chloride. Silver(I) chloride is very insoluble, so it will precipitate, leaving soluble sodium nitrate in solution. Precipitation reactions can be a good way to prepare a salt you want from some other salts with the right anion and cation. Precipitation reactions can also be used to detect the presence of particular ions in solution. For instance, you might test for chloride, iodide and bromide in an unknown solution by adding silver(I) ions and looking for precipitation.
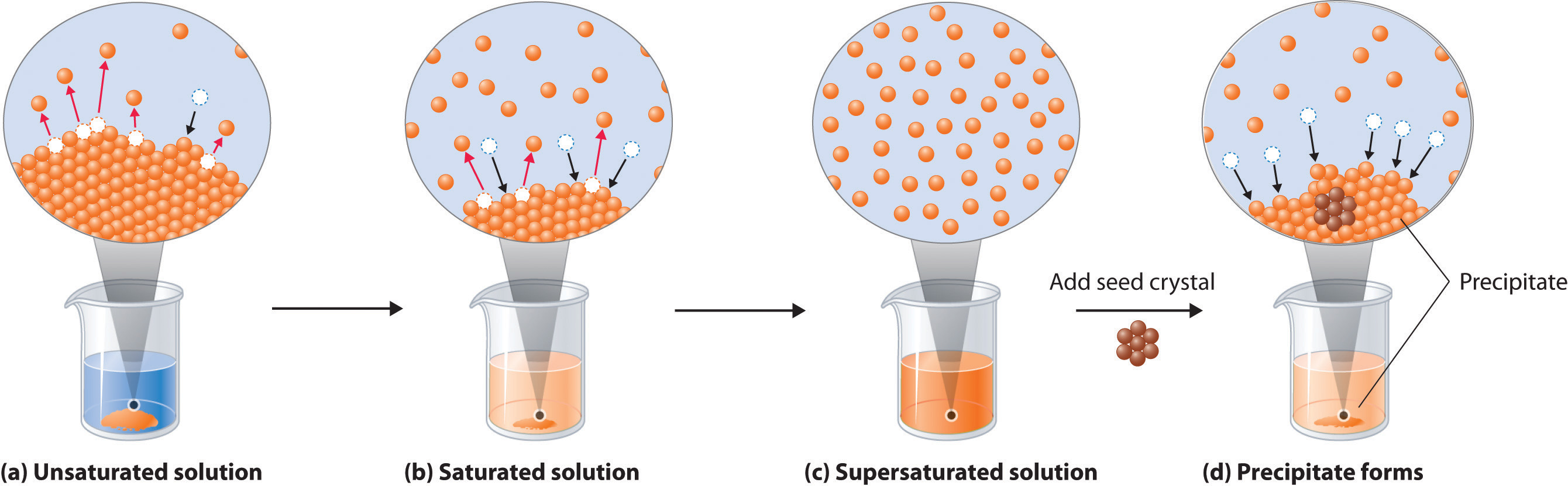
Predicting Precipitation Reactions
Beginning chemistry students usually memorize a list of solubility rules. Here it is (these rules will be a little bit different in different textbooks, because people might not have exactly the same definition of soluble or insoluble):
- Most nitrate and acetate salts are soluble
- Most alkali cation and ammonium salts are soluble
- Most chloride, bromide and iodide salts are soluble, except those of Ag(I), Pb(II) and Hg(I)
- Most sulfate salts are soluble, except those of barium, calcium and Pb(II)
- Most hydroxide salts are only slightly soluble, except those of sodium and potassium
- Most sulfide, carbonate and phosphate salts are only slightly soluble
You can use this list to predict when precipitation reactions will occur. For this purpose, you don't usually have to worry about whether the compounds are strong or weak electrolytes, you can think of the ions as being separate. The reason is that usually some of the ions will be separate, and once those precipitate with a new partner, more of the original compound ions will separate from each other, and the process will continue.
Writing Equations for Precipitation Reactions
Chemists may write equations in different ways to emphasize the important parts. For instance, we might write an equation like this, which describes mixing 2 solutions of different soluble salts and getting a precipitate:
$$AgNO_{3}(aq) + NaCl(aq) \rightarrow AgCl(s) + NaNO_{3}(aq)$$
Alternately, we might write the same reaction just focusing on the part that forms the precipitate, and leaving out the spectator ions that don't really do anything, just stay in solution:
$$Ag^{+}(aq) + Cl^{–}(aq) \rightarrow AgCl(s)$$
Chemistry students are sometimes asked to prove their understanding of dissociation by writing out all the ions separately, like this:
\[Ag^{+}(aq) + NO_{3}^{–}(aq) + Cl^{–}(aq) + Na^{+} \rightarrow AgCl(s) + Na^{+} + NO_{3}^{–}(aq)\]
No real chemist would be likely to do this because it is a nuisance. (It's also a little funny because many salts aren't strong electrolytes, so teachers might be telling their students to write an equation that doesn't show what's really happening.) However, it does help show what it means to be a spectator ion, since they are the same on both sides when you write it like this.
What Determines Solubility?
Solubility depends on the relative stability of the solid and solvated states for a particular compound. For instance, if it has very strong interactions between molecules or ions in the solid state, then it won't be very soluble unless the solvation interations are also very strong. (Ionic salts are a good example: usually they have strong interactions in the solid and solvated states.) If the interactions in the solid are weak, the compound can still be insoluble in polar solvents if the interactions with the solvent are weaker than the Coulomb interactions of the solvent molecules with other solvent molecules. (This is why wax is insoluble in water: it is non-polar, so the wax-wax interactions are weak, but the wax-water interactions are weaker than the water-water interactions.) We can't explain what makes these interactions strong or weak well until after we study chemical bonding, but in general ionic compounds with larger charges on the ions and smaller ions are less soluble, because they can have stronger Coulomb interactions in the solid.
Outside Links
- Solubility Explained (13 min)
- CrashCourse Chemistry: Precipitation (12 min)
Contributors and Attributions
Emily V Eames (City College of San Francisco)

