1.4: Collisions and Phase
- Page ID
- 189835
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Reaction rates depend on the energy required (the activation barrier) and the energy available. They also may involve collisions between molecules.
If two molecules need to collide in order for a reaction to take place, then factors that influence the ease of collisions will be important.
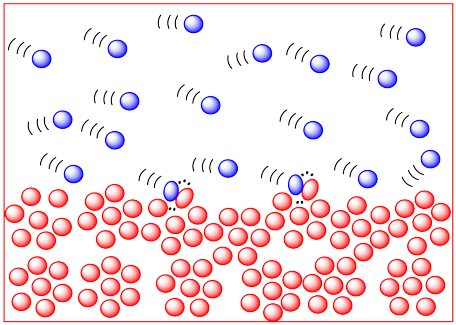
The more energy there is available to the molecules, the faster they will zip around. The faster they zip around, the more likely they are to bump into each other. So higher temperatures ought to lead to more collisions and a greater frequency of reactions between molecules. In the drawing below, the cold, sluggish molecules on the left don't appear to be in danger of colliding with anything, but the hot, zippy molecules on the right look like they are due for a crash at any time.
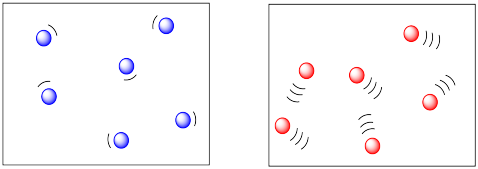
We already knew that higher temperatures increased reaction rates. This new observation is just an additional reason why temperature is important in kinetics.
- Temperature affects molecule mobility
- The higher the temperature, the more mobile the molecules will be, and the more likely they are to collide and react.
Phase also has a pronounced effect on the mobility of molecules. Molecules in the gas phase are quite free to move around, and they do so pretty quickly. On the other hand, they are pretty well spread out. Nevertheless, collisions in the gas phase happen pretty easily, which might help gas-phase reactions happen more readily.
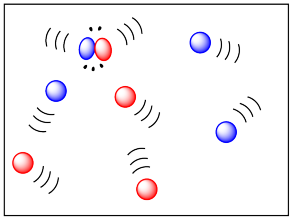
At the opposite extreme, molecules in the solid phase are not very mobile at all. (Reactions may involve atoms or ions, rather than moleules, but the same arguments apply.) Not many collisions happen. As a result, reactions often happen extremely slowly in the solid state. Reactions are mostly limited to the grain boundaries: the surfaces of the grains, where they are in contact with each other. Nothing happens in the middle of a lump of solid, which remains unreacted.
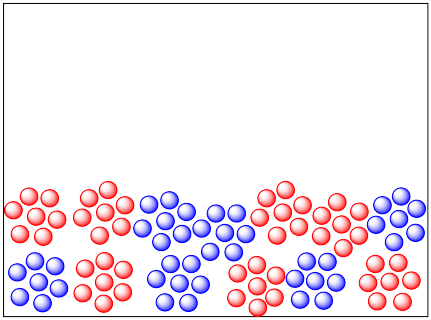
If you heat a solid up, the molecules can move around a little more. They may even leave their crystal lattice (if the solid is a crystalline one) and diffuse very slowly through the solid. Many solid state reactions are run at elevated temperature.
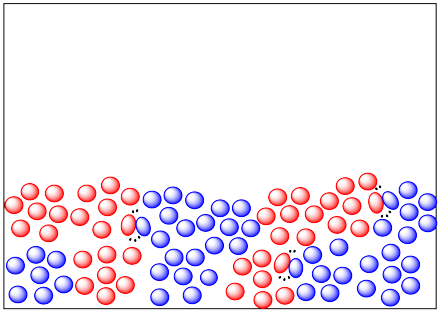
There are also many solid state reactions that are conducted in combination with gas-phase reactants. The solid reactants are often heated in a furnace while gas-phase reactants flow over them.
Many reactions are performed in the liquid state, either because the reactants are already liquid of because the solid reactants are heated past their melting point. In the liquid phase, molecules are much more mobile and collisions are much more frequent than in the solid phase.

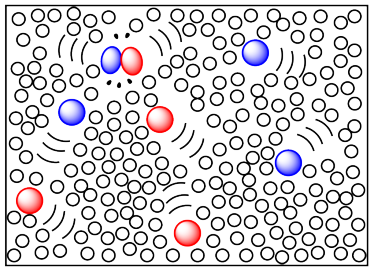
It is also very common to run reactions in solution. In solution, a compound that is meant to undergo reaction is dissolved in a solvent. The solvent needs to be a liquid at the temperature at which the reaction will be run, so that molecules will be very mobile, but will still be close together, so collisions are favored.
There are many advantages to running reactions in solution. The reactant molecules are very mobile and pretty close together, so that collisions are facilitated. If the reaction is exothermic and gives of a lot of heat, the excess heat can be absorbed by the solvent molecules and carried away. That can be important in controlling reactions and avoiding decomposition. Also, we will see that the rate of collisions can be controlled by adding more solvent or less, in order to slow the reaction down or speed it up. In this way, the reaction rate can be controlled to some extent.
There are many liquids that are commonly used as solvents. Dichloromethane, toluene, dimethylformamide, tetrahydrofuran and acetonitrile are some common "organic" solvents, so called because they are based on carbon, which forms the basis of molecules in organisms. These different solvents offer a range of polarities, so different ones can dissolve different reactants.
Water may be the most common solvent on the planet, and it is non-toxic, so it is very appealing for use in large-scale, industrial reactions. However, it is not very good at dissolving non-polar reactants.
The reactant compound could be a liquid or a solid. It just has to have strong enough intermolecular attractions with the solvent molecules so that it can become dissolved. Individual molecules of the reactant become lost among the solvent molecules and swim around with ease.
The disadvantage of using a solvent is that the solvent must be removed at the end of the reaction, so that the desired product can be isolated and used. That means the solvent may eventually be thrown away as waste. That practice is less efficient and less environmentally friendly, although the solvent could possibly be recycled.
One method of dissolving reactants that is potentially greener is the use of supercritical fluids. In this approach, gases such as carbon dioxide are pressurized until they turn into liquids. In this form, carbon dioxide is a pretty good solvent, and reactions can be run when reactants are dissolved in it. At the end of the reaction, a valve is opened, releasing the pressure, and the carbon dioxide turns back into a gas. It can be stored and re-pressurized for another reaction.
Diffusion in the solid state is greatly enhanced by defects in the crystal lattice. Show why with the following drawing.
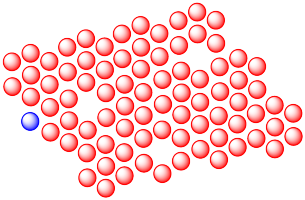
Answer-
The vacancies in the crystal lattices give other atoms places to move into, so they act as a path through which atoms can move. Diffusion through the solid is greatly accelerated.
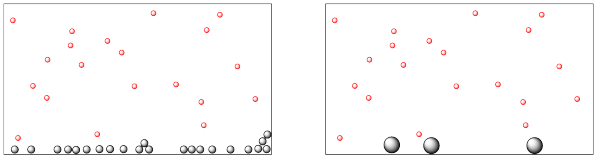
The following drawing represents a reaction between molecules in the gas phase and molecules in the solid phase.
- Which material is in the gas phase and which one is in the solid phase?
- Both pictures contain the same mass of each material. One of these materials appears to be distributed differently in each picture, however. Explain how that difference may affect the reaction rate.
- Answer a
-
The small, red dots are in the gas phase, so they are distributed throughout the container. The larger, gray dots are the solid, which lies along the bottom of the container.
- Answer b
-
The solid on the left is divided into finer particles, with much more surface area. If the gas reacts on the surface of the solid, reaction will be much faster on the left.
Three common solvents are shown below. Compare and contrast these three solvents in terms of how they interact with other molecules.
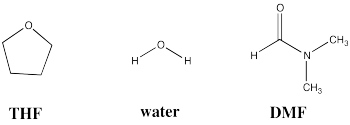
- Answer
-
All three of these solvents have dipole moments. Also, all three molecules have oxygen lone pairs, so they are able to accept hydrogen bonds from hydrogen bond donors.
However, some of the solvents are much more polar than others. Water is capable of donating hydrogen bonds, because of its partially positive hydrogen attached to oxygen. It is the most polar of these solvents.
DMF is also very polar, because it has a polar C=O bond. This particular carbonyl is more like +N=C-O- because of lone pair donation from the nitrogen, so it is quite polar and will interact strongly with other species via dipole-dipole forces (or ion-dipole forces, if the other molecule is a salt).
THF only has a moderate dipole compared to the others. Although it will still interact via dipole-dipole (or ion-dipole) forces, it does so less effectively than water or DMF.
Indicate how well you think each of the three solvents in the above question (THF, water, DMF) could dissolve each of the following compounds. Justify your answers in terms of interactions between molecules.
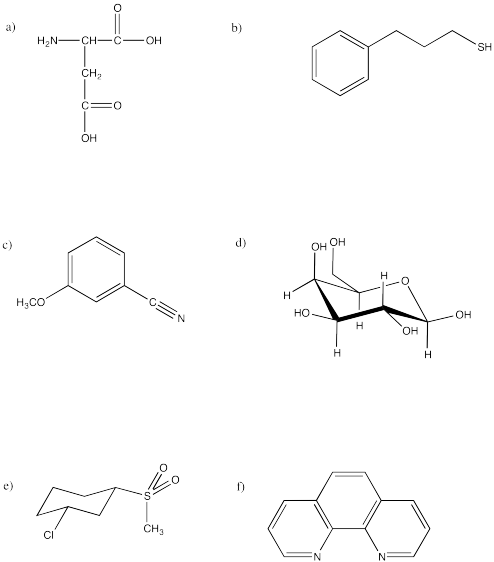
- Answer
-
Water would be a very good solvent for (a) and (d), because both of those molecules would be very good at hydrogen bonding. Although water may be able to dissolve small amounts of the others, their solubility would be limited by the need for water molecules to release hydrogen bonds to each other in order to make room for the non-polar portions of these molecules.
THF would be able to dissolve the other molecules pretty well: (b), (c), (e) and (f). All of those molecules contain polar bonds, like THF, and could interact via dipole-dipole forces. THF would be able to dissolve small amounts of (a) and (d) but may not be polar enough to overcome the stronger intermolecular forces between these molecules.
DMF may be able to dissolve all of these molecules to a moderate extent. Although it is not a protic solvent, its dipole is enough to help overcome hydrogen bonding among (a) and (d).


