5.12: Condensation Polymers and Ring-Opening Trans-Esterification Polymerisation
- Page ID
- 199677
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Carboxyloids, such as esters, can interconvert with each other in the presence of the appropriate nucleophile. In the case of esters, an equilibrium will result. If an alcohol is added to an ester, under the right conditions we might get a new ester. A new alcohol would also appear, originating from the old OR group of the original ester. Two different esters and two different alcohols would be in equilibrium. This equilibrium might be perturbed in one direction or another, for example, by the addition of an excess of one nucleophile.
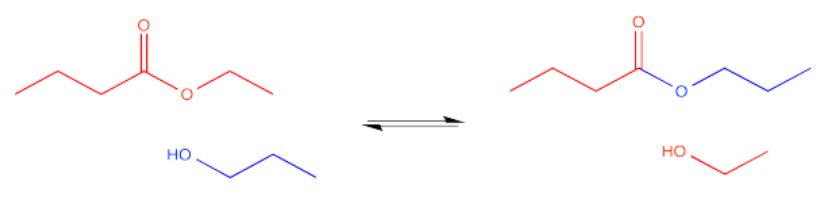
In the jargon of synthetic organic chemistry, an ester is a functional group. It is a site on the molecule at which reactions take place. It is also a site on the molecule that is easily subject to synthetic transformations. In other words, one functional group might easily be converted into another. In the case of a trans-esterification reaction, one ester simply gets converted into another.
Some compounds have more than one functional group. A compound could be both an ester and an alcohol, for instance. We might call this compound a hydroxyester. But wait -- if an alcohol can react with an ester, to make a new ester, doesn't this compound have both components of a reaction built in? Could it react with itself?
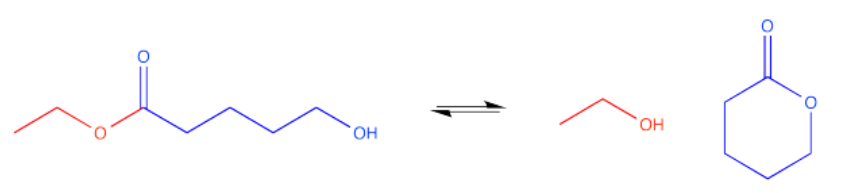
There are a couple of ways that could happen. If the chain between the carbonyl and the hydroxyl group is long enough (remember, a six-atom interaction between the hydroxyl oxygen and the carbonyl carbon may be optimal), the hydroxyl could wrap around and form a cyclic ester. That's an intramolecular reaction -- a reaction within one molecule.
Alternatively, if there is another one of these molecules around someplace, an intermolecular reaction might occur. That's a reaction between two different molecules. The hydroxyl group on one molecule can reach out and react with the carbonyl on another molecule.
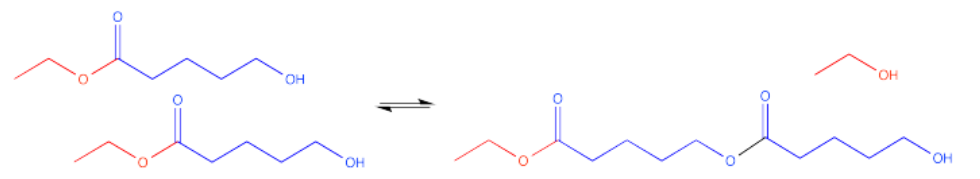
Now there are two molecules of the same kind bonded to each other. This double molecule is called a dimer. The individual molecules that have been linked togethr to make the dimer are called monomers.
That dimer has two esters in it, not just one. Of course, it still has a hydroxyl group on one end. That hydroxyl group can still react with another carbonyl on another molecule.
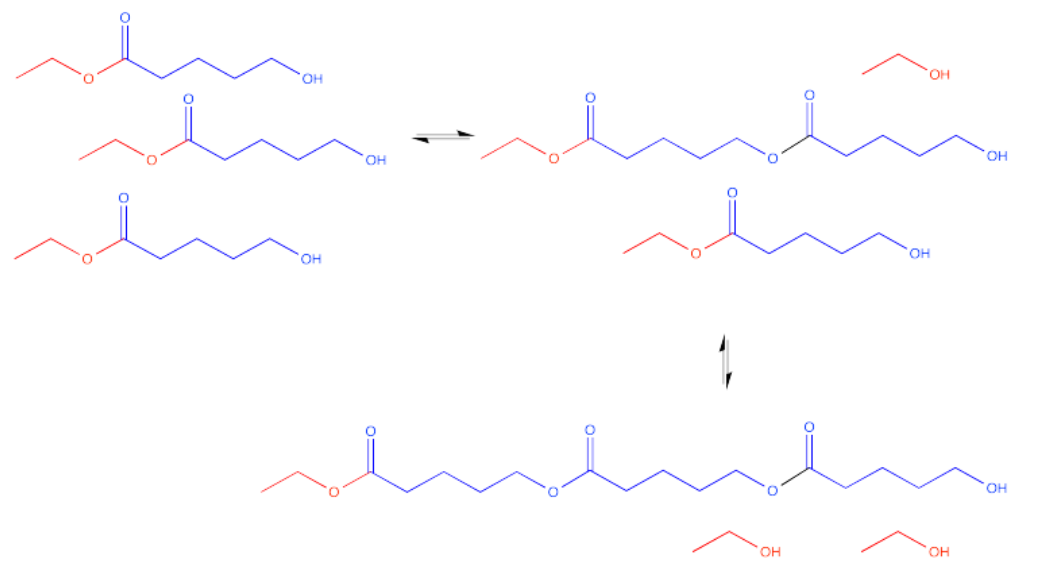
Now there are three molecules bonded together. This molecule is called a trimer.
This process could keep going on indefinitely, of course. We might end up with a very large molecule, composed of many individual (former) molecules that have bonded together. This very large molecule made up of repeating units is called a polymer. A polymer is built up from many monomers linked together. This particular kind of polymer is called a polyester.
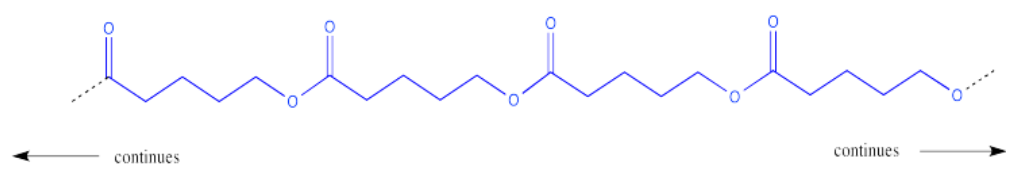
This polymer is frequently drawn in a way that emphasizes the repeating pattern of monomers that have been incorporated into the chain.
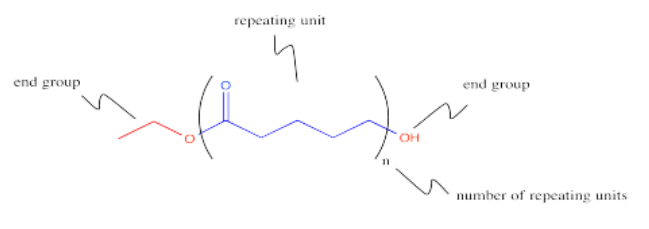
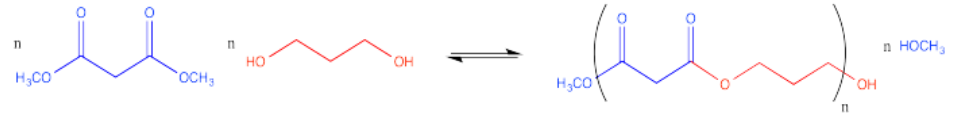
Polyesters can also be made by co-polymerizing two different monomers together. One monomer could be a diester, for example. The other monomer could be a diol. These two molecules are ready to react together, with one molecule acting as an electrophile and the other molecule acting as the nucleophile.

Together, the diester and the diol could be polymerized. The result would be an alternating copolymer, in which diester and diol monomer units alternate all along the polymer chain.
Polymers make up an important class of materials with many uses. Many polymers are lightweight, strong materials used to make parts for automobiles and other products. Polymers can also be very flexible or elastic. The physical properties of polymers are very different from the properties of other molecular compounds. These differences are a direct result of the very large size of polymer molecules. A polymer molecule might be thousands of monomers long, with a molecular weight in the millions.
Exercise \(\PageIndex{1}\)
Problem CX8.1.
Express the following polymer structures in abbreviated structures showing n repeating units in parentheses.
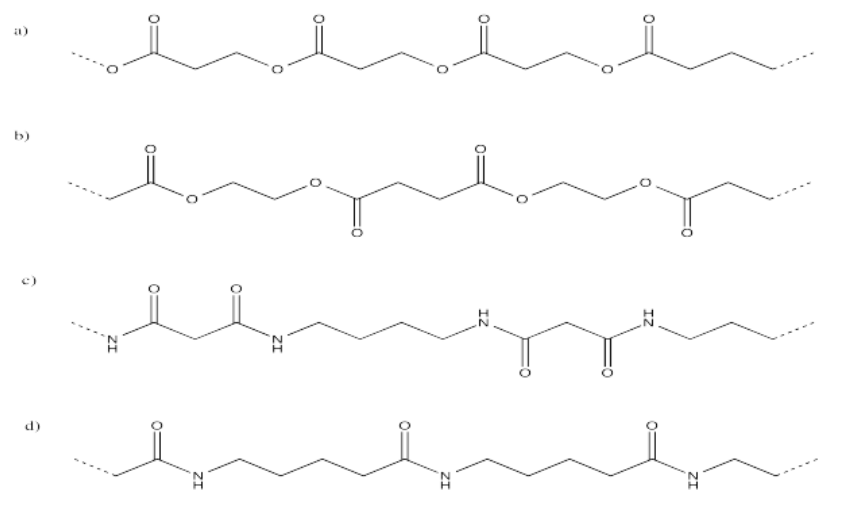
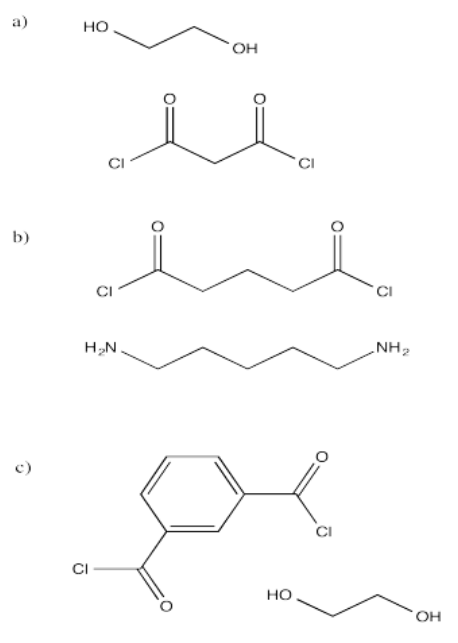
Exercise \(\PageIndex{2}\)
Show the structures of the polymers that would result from the following monomers. In each case, show a drawing with several enchained monomers.
Exercise \(\PageIndex{3}\)
Ring-opening polymerization involves a multi-step reaction in which a cyclic compound, such as a lactone (below) is opened into a chain through the addition of a nucleophile (called the "initiator"). The resulting chain is able to act as a nucleophile and open the next lactone, and so on, until a polymer has formed. Show a mechanism for formation of the oligomer in which n = 3.
Exercise \(\PageIndex{4}\)
Ring-opening polymerizations are frequently accelerated through the addition of small amounts of metal compounds, such as diethylzinc (Et2Zn) or tin octoate (Sn(O2CCH(CH2CH3)CH2CH2CH2CH3)2. Explain the role of these compounds in the reaction mechanism.
Exercise \(\PageIndex{5}\)
Karen Wooley at Texas A&M recently reported the following synthesis of a polyphosphoramidate for use as a pharmaceutical delivery agent. The goal is to use a benign delivery agent that is easily broken down and excreted by the body, resulting in low toxicity and minimal side effects.
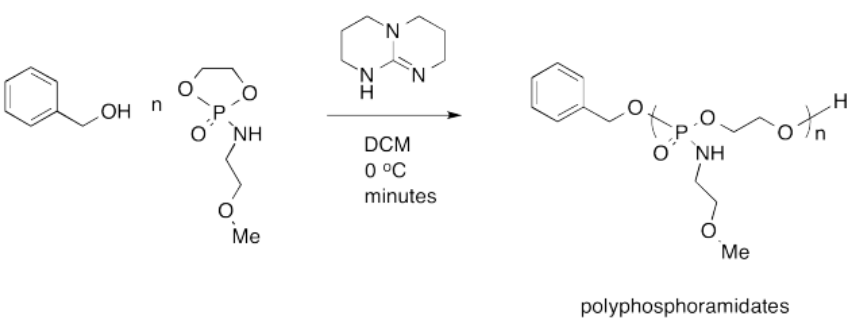
- Provide a mechanism for the synthesis.
- Explain why the polyphosphoramidate is expected to be broken down and excreted easily by the body.
In ring-opening polymerisation, the monomer is not difunctional. Instead, it is embedded in a ring. Ideally, there is a little ring strain in the molecule, bumping it up in energy just a little so that it will react more easily. Common examples include caprolactone and lactide, used to make biodegradable yard waste bags and produce containers, respectively (among many other applications). These cyclic esters are sometimes referred to as "lactones".
If an alcohol is added, it can act as an "initiator" in a "chain reaction". The alcohol is a nucleophile, and it donates to the carbonyl, eventually cleaving the carboxyl C-O bond and popping open the ring.
At some point, a proton gets transferred to the oxygen that used to be embedded in the ring. Now we have a new alcohol. What does it do? It reacts with another cyclic ester, popping it open and forming a new alcohol. The cycle repeats itself.
- A chain reaction keeps happenning over and over again.
- A chain reaction must be started by an initiator.
- A chain reaction leads to a product that looks just like an earlier reactant, so the product reacts again.
In reality, ring opening polymerisations don't really work if you just add an alcohol to a lactone. Typically, a catalyst is also added. Catalysts most commonly are Lewis acids, such as aluminum, iron or tin compounds. One of the most common catalysts is tin octoate, more properly called tin(II) 2-ethylhexanoate.
Exercise \(\PageIndex{6}\)
Provide a mechanism, with arrows, for the ring-opening polymerisation of caprolactone with tin octoate.
Exercise \(\PageIndex{7}\)
Perform end-group analysis in the following cases to determine
- the degree of polymerisation (what is the value of "n"?).
- the molecular weight.
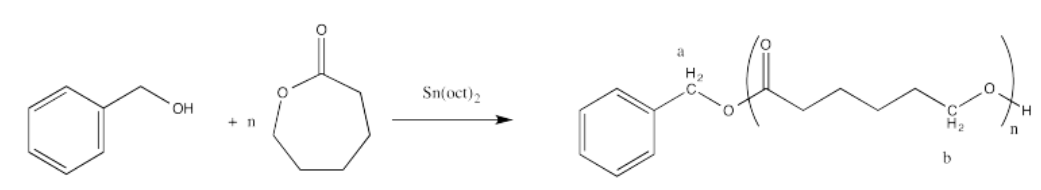
i. The ratio of the integrals for the 1H NMR peaks representing positions b:a is 50:1.
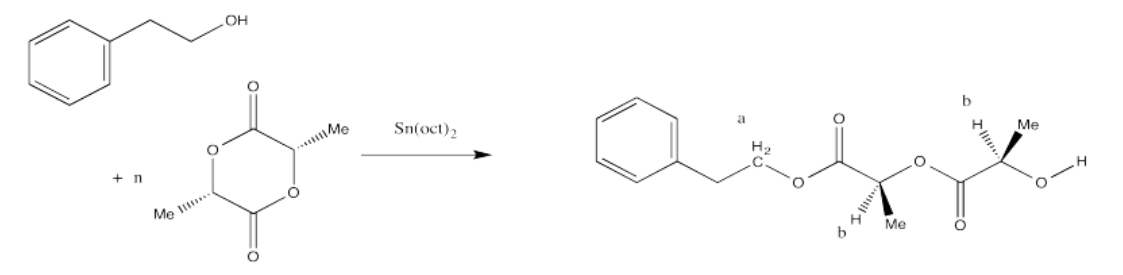
ii. The ratio of the integrals for the 1H NMR peaks representing positions b:a is 80:1.
Answer i-
In general, we compare a peak in the repeat unit (which occurs over and over) to a peak in an end group (which occurs only once in each chain) to find the number of repeat units or degree of polymerization. For example, if the chain were a dimer (n = 2) we would expect the integral for peak b to be twice as large as the integral for peak a.
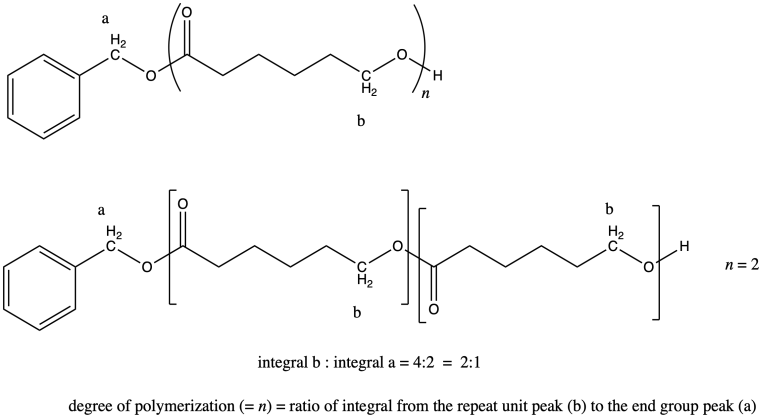
It works out that way because in each repeat unit, peak b represents two hydrogens, and in the end group, peak a represents two hydrogens. It's the same number of hydrogens in each position, so comparing the integration of the two peaks tells you directly how many repeat units there are.
If that were not true (if peak b represented only 1H and peak 1 represented 2 H), then we would have to factor that difference into the answer.
The question states that the integration ratio of b:a is 50:1, so that means the degree of polymerization = 50.
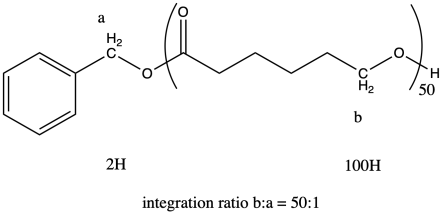
The molecular weight is therefore about 50 times the molecular weight of the monomer (114.4 g/mol), or 5,720 g/mol (sometimes expressed as 5,720 Da; a Dalton is just 1 g/mol). There are also end groups (from benzyl alcohol) that contribute a little weight, so the total molecular weight is 5,720 + 108 g/mol = 5,828 g/mol.
- Answer ii
-
Once again, peak a represents two hydrogens, and so does peak b (although the peak b hydrogens occur at two different places in the molecule and differ stereochemically from each other). That means that, once again, the ratio of integrals of peak b to peak a tells us the degree of polymerization. If the ratio were 3:1, we would have n = 3.
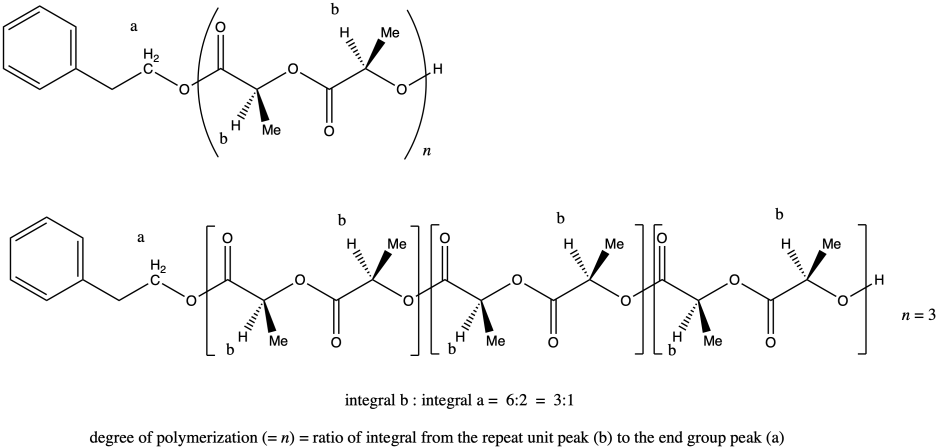
The question states that the integration ratio of b:a is 80:1, so that means the degree of polymerization = 80.
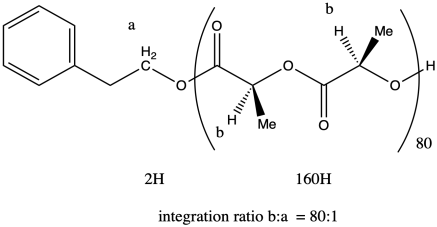
The molecular weight is therefore about 50 times the molecular weight of the monomer (144.1 g/mol), or 11,530 g/mol. Adding in the end groups (from benzyl alcohol), the total molecular weight is 11,530 + 108 g/mol = 11,638 g/mol.
Note that in certain diastereomers of lactide (LLA or DLA) we could actually draw the repeat unit as a C3H4O2 unit rather than as the larger C6H8O4 unit, but in this case the two chiral centers are actually different, so we have to keep the larger repeat unit to show the presence of both stereochemical configurations.


