5.11: Enolates - Claisen Condensation and Decarboxylation
- Page ID
- 199381
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alkylmagnesium reagents, alkylcuprates and complex hydrides can all react with carboxyloids. When they do, a carbon or hydrogen nucleophile bonds to the carbonyl carbon, usually replacing the leaving group at that position.
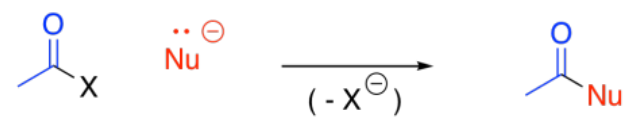
Another common carbon nucleophile is an enolate ion. Enolate ions can also react with carboxyloids, although not typically with amides.
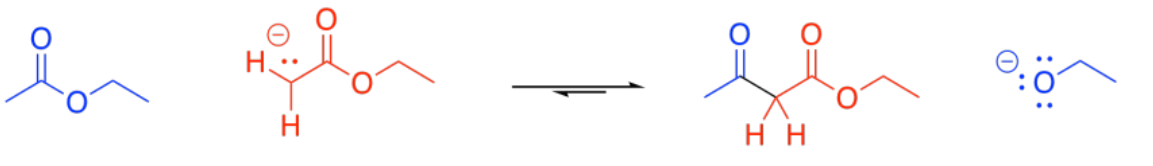
Probably the most common enolate reaction involving carboxyloids is the reaction of esters. If a strong base is added to solution of ester, some of the esters will become deprotonated, forming enolate anions. These ions will be nucleophiles.
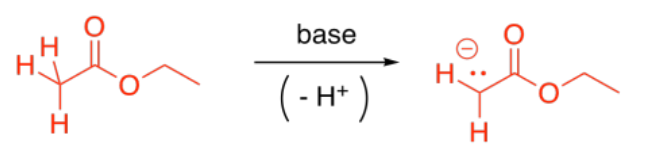
Some esters will remain protonated. These esters will be electrophiles. Donation of the enolate to the ester, with subsequent loss of the leaving group, leads to a beta-ketoester.
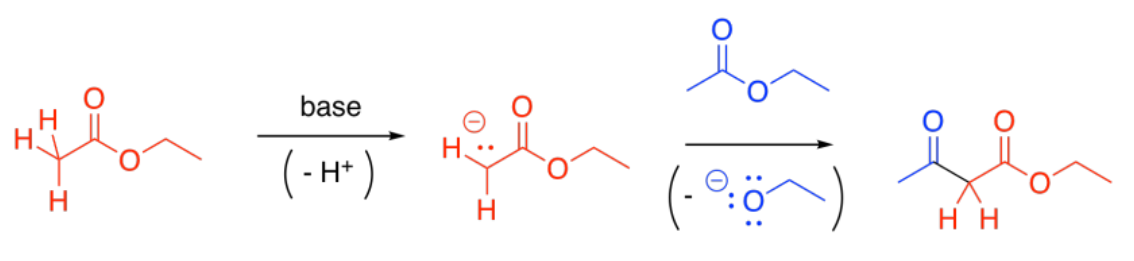
You are familiar with the term "alpha-position". That's the position next to a carbonyl. The "beta-position" is the next one after the alpha position. In a beta-ketoester, there is a ketone in the beta position of the ester. The formation of a beta-ketoester from two esters is called a "Claisen condensation".
In principle, this reaction could conceivably go backwards. The enolate ion could potentially be displaced by an alkoxide to get back to an ester and an enolate ion. That's because the enolate ion is a relatively stable ion, and a moderately good leaving group. However, that generally doesn't happen.
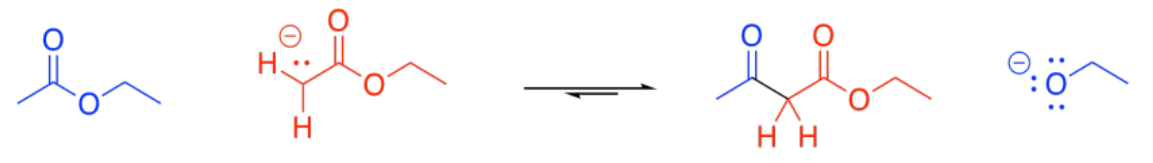
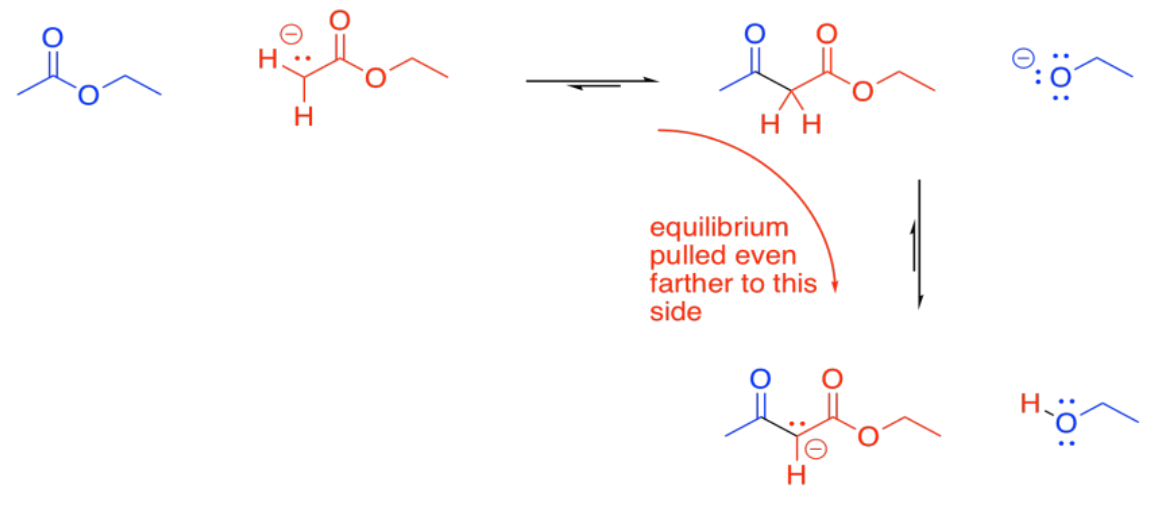
Under basic conditions, the beta-ketoester is usually deprotonated, forming a particularly stable ion. This ion formation acts as a "thermodynamic sink" for the reaction, pulling it forward until all of the ester has been consumed.
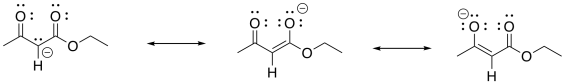
Exercise \(\PageIndex{1}\)
Show why the ion that results from deprotonation of the beta-ketoester is particularly stable.
- Answer
-
Exercise \(\PageIndex{2}\)
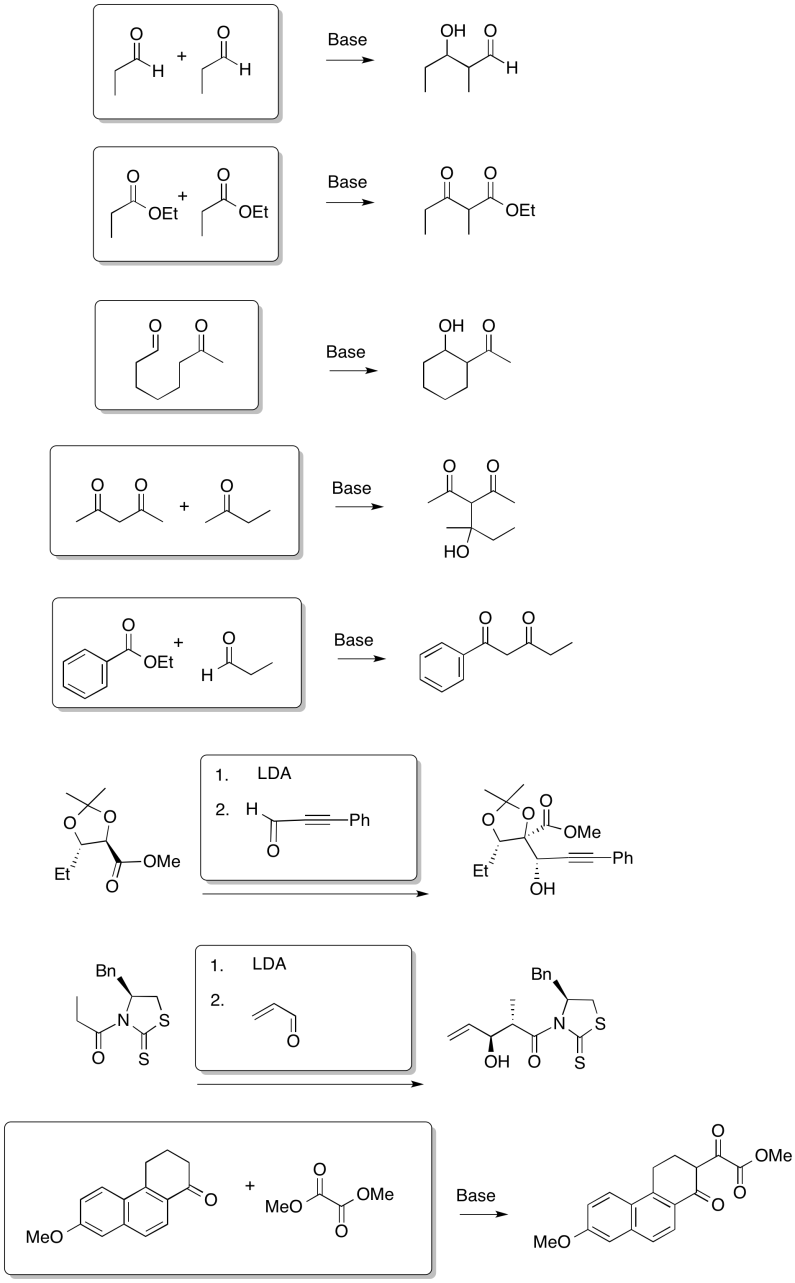
Fill in the products of the following reactions.
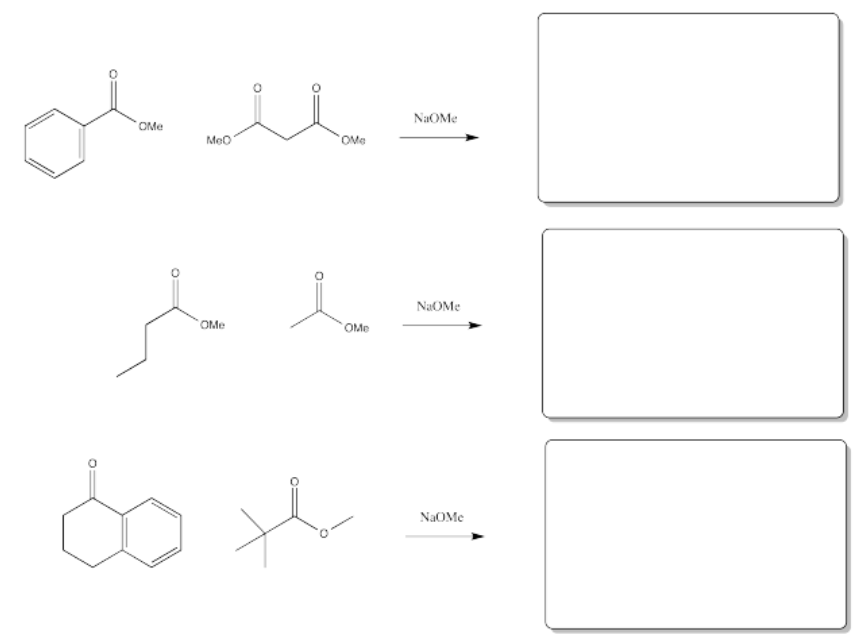
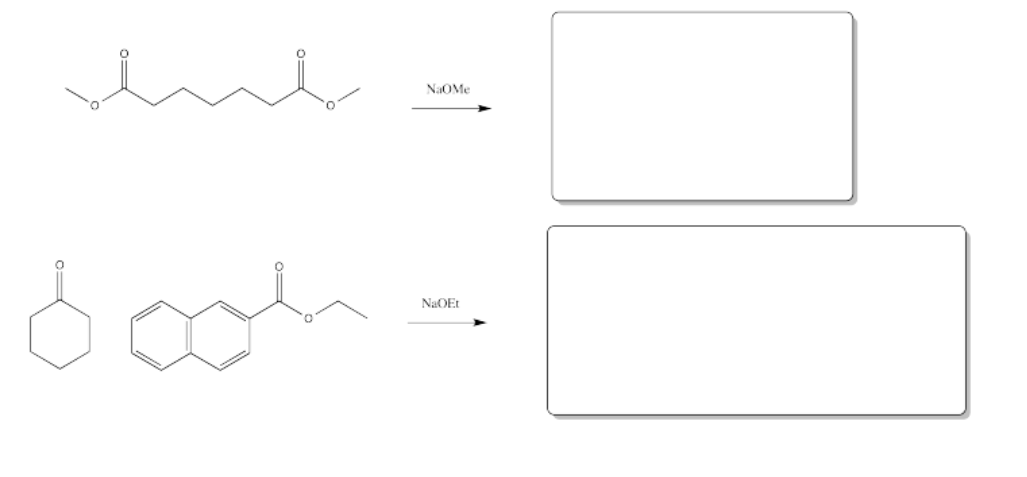
Answer-
Exercise \(\PageIndex{3}\)
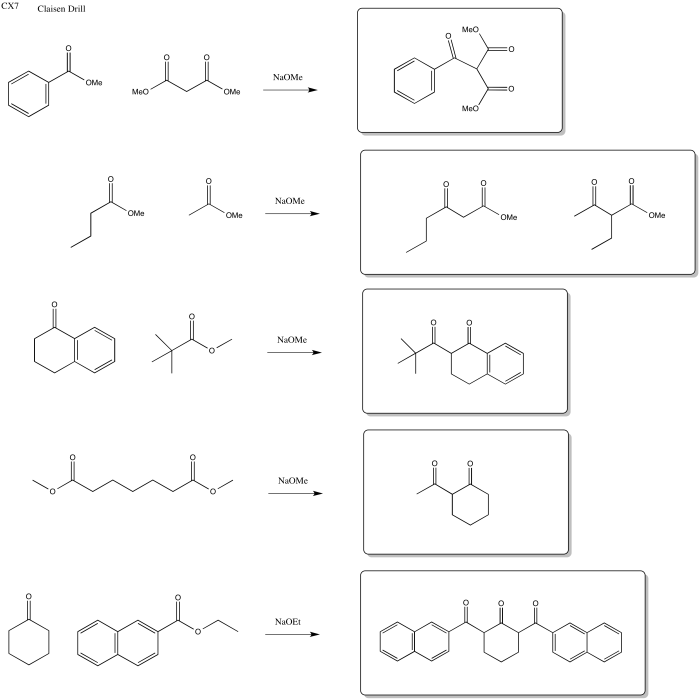
Predict the reactants needed to make these products via a Claisen, Aldol or Crossed Aldol reaction.
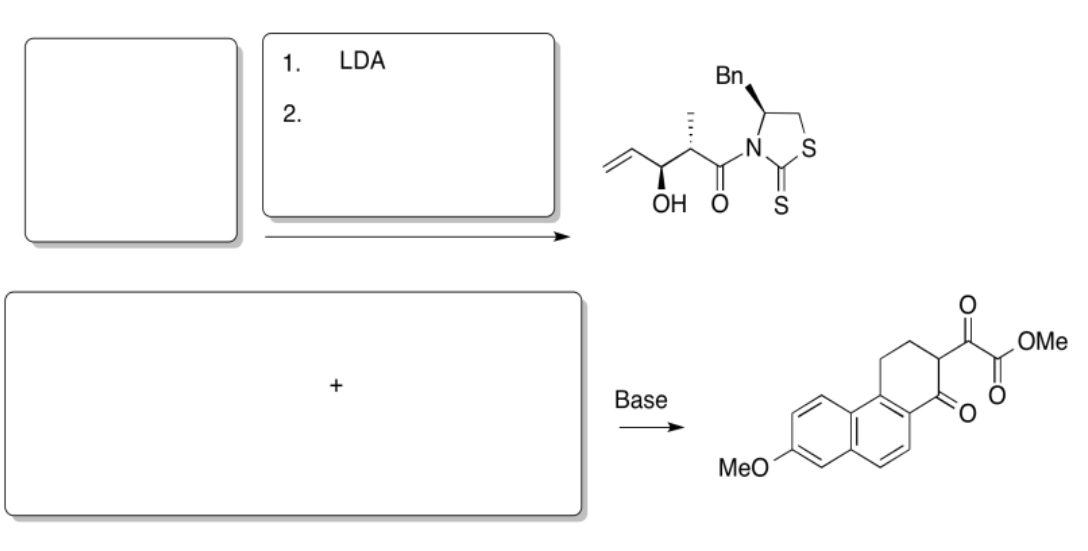
Answer-
The formation of a beta-ketoester from two esters is called a "Claisen condensation".
It is often followed by another important reaction: decarboxylation. If a beta-ketoester is treated with aqueous acid and heated, a couple of reactions take place. First, the ester portion of the molecule is converted into a carboxylic acid.
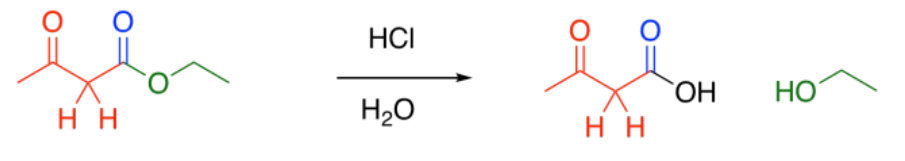
Second, the carboxylic acid is decarboxylated. Carbon dioxide is formed, and the organic molecule becomes a ketone. The carboxyl group is lost completely from the original molecule, and is converted into CO2.
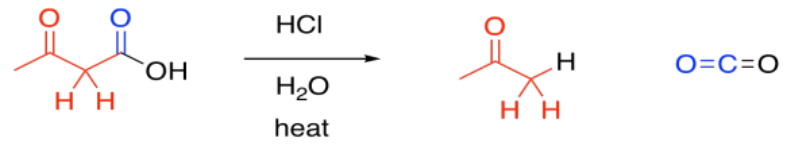
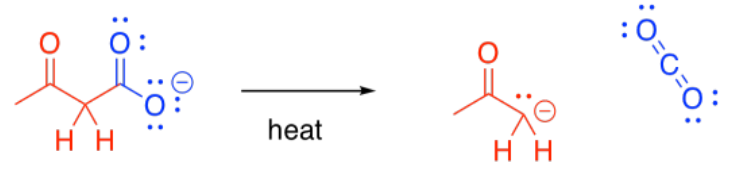
Decarboxylation is related to the retro-aldol reaction; formally, it can be thought of as leading to an enolate leaving group. Decarboxylation most commonly occurs in beta-ketoacids, rather than in other carboxylic acids. Otherwise, that leaving group could not occur. The ease of decarboxylation in beta-ketoacids is related to the stability of the enolate anion.
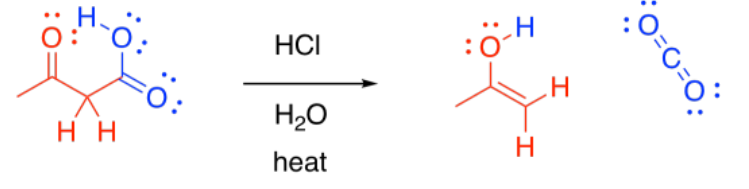
Under acidic conditions, of course, an enolate anion does not occur; instead, an enol is formed. However, enols are rapidly converted into the keto tautomers.
Exercise \(\PageIndex{4}\)
Draw a mechanism for:
- Conversion of ethyl-3-oxyhexanoate into 3-oxyhexanoic acid. (Oxy is a prefix meaning a ketone or aldehyde is foundalong the chain).
- Decarboxylation of the resulting 3-oxyhexanoic acid.
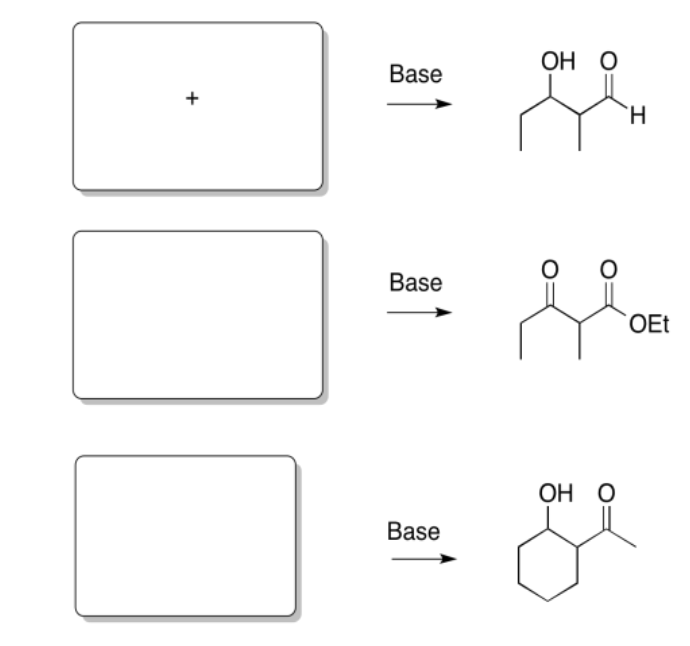
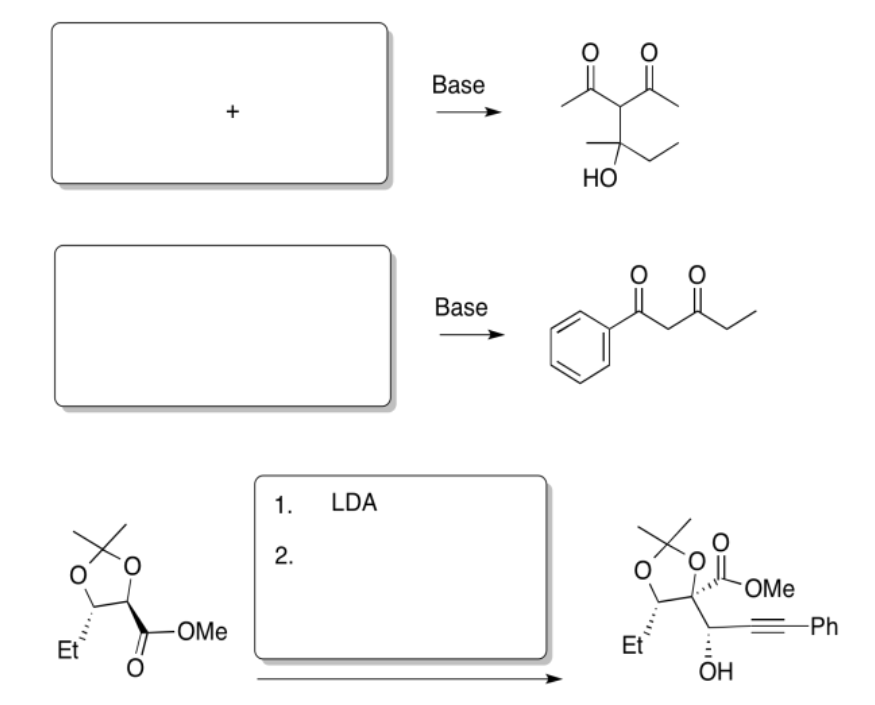
Exercise \(\PageIndex{5}\)
Fill in the products of the following reactions.
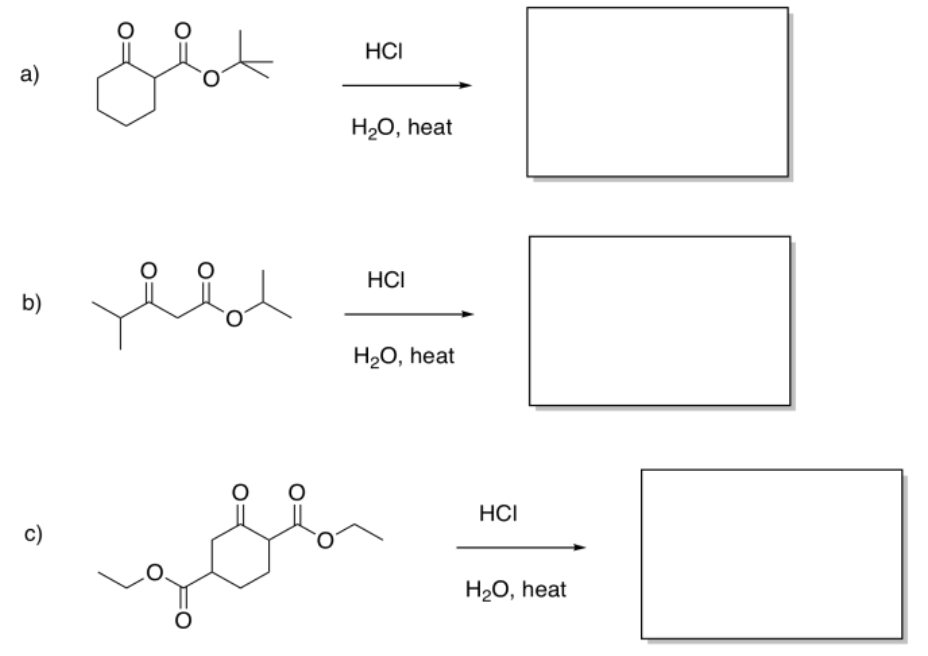
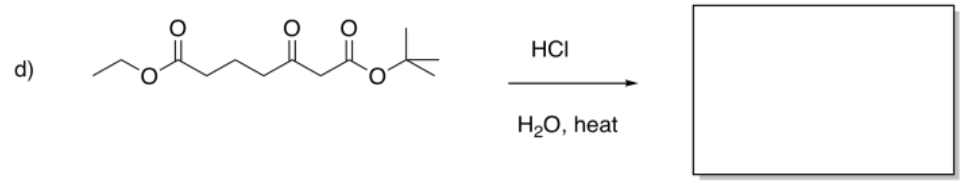
- Answer
-