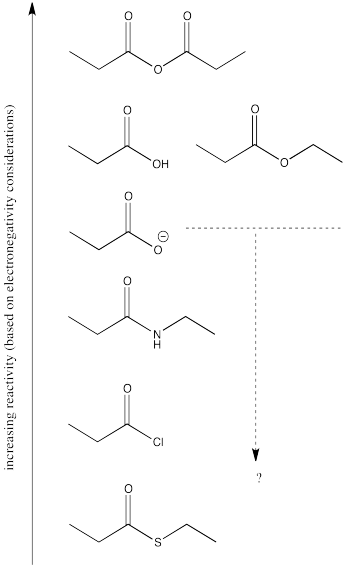
5.7: Comparative Energies- The Ski Hill
- Page ID
- 189948
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In most cases, the reactivity of carboxyloids involves converting one carboxyloid into another. This is done by replacing one heteroatom substituent with another. For example, the chloride in an acid chloride may be replaced by an alcohol or alkoxide ion to make an ester.
The fact that one carboxyloid can be converted into another suggests that there would be an equilibrium between them. The ratio of two carboxyloids at equilibrium would be determined by their relative stability, as well as the stability of other associated species in solution.
We can map out the stability of carboxyloids on a potential energy surface, as shown below. The higher energy, less stable, more reactive carboxyloids are shown at the top of the potential energy curve. The lower energy, more stable, less reactive carboxyloids are found lower down on the potential energy curve.
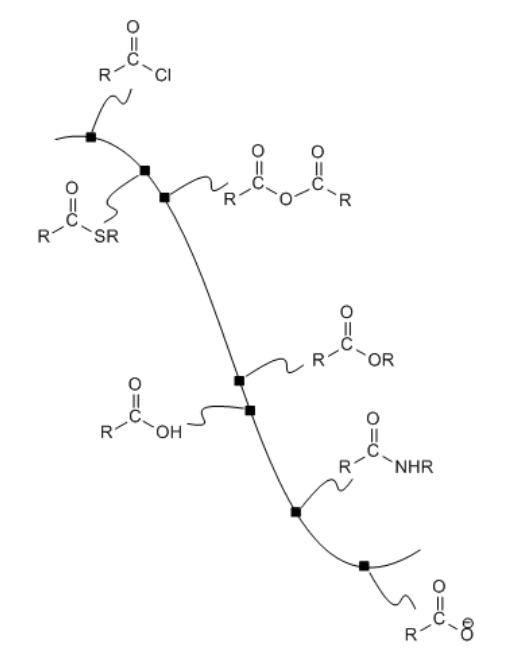
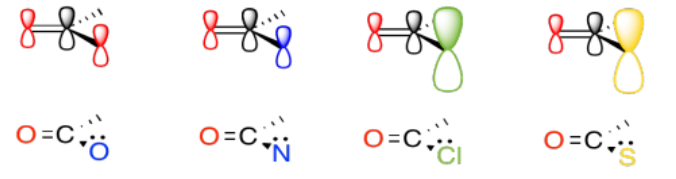
The heteroatom attached to the carbonyl in a carboxyloid is always an electronegative atom with a lone pair. Either of those two features might be useful in understanding the reactivity trend illustrated above. For example, an electronegative atom would make the carbonyl carbon more positive. That carbon is already very positive because of the double bond to oxygen. Adding an additional electronegative atom should make it even more so. The amount of positive charge on the carbonyl carbon would be even greater if the atom attached to it were exceptionally electronegative.
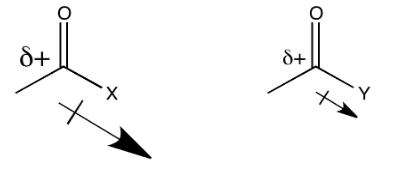
On the other hand, a nearby lone pair might counteract the electron-attracting power of the carbonyl carbon. In a sense, we might think about that lone pair as competing with donation from a potential nucleophile.
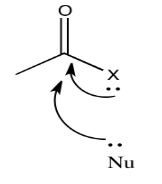
The ability of an atom to π-donate, then, might have an influence on how strongly the carbonyl will attract nucleophiles. Of course, there is some trade-off involved in π-donation. Usually the atom that donates must take on a positive charge, since it is lending a pair of its own electrons to another atom. Factors that influence how easily this may happen could be important in determining carboxyloid reactivity.
Exercise \(\PageIndex{1}\)
Based on electronegativity of the atom attached to the carbonyl carbon, we might expect a specific trend in carboxyloid reactivity. Explain how this factor would affect electrophilicity at the carbonyl carbon and predict the corresponding trend in reactivity. Compare this trend with the information in Figure \(\PageIndex{1}\) (CX3.1).
- Answer
-
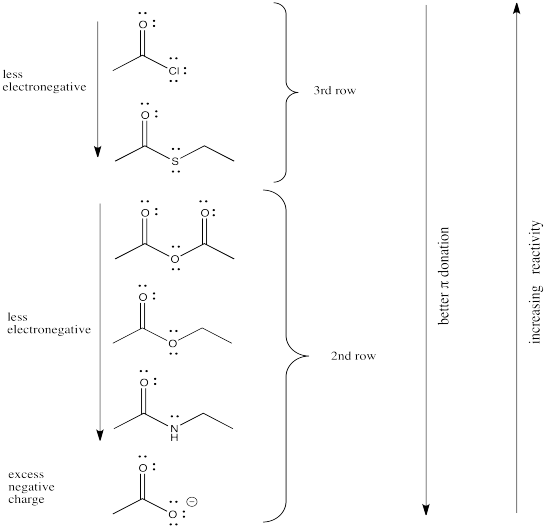
We might expect carboxyloids with the most electronegative elements attached to the carbonyl to be the most reactive and least stable towards substitution (in other words, carboxyloids with the most electronegative heteroatoms would become substituted the most easily).
In that case, we would predict that the carboxyloids with the most electronegative substituent (oxygen) would be the most reactive. There are a number of different kinds and we will think about how they relate to each other shortly.
After the oxygen derivatives we would predict either the nitrogen derivatives or the chloride, depending on what electronegativity scale we happen to use (remember, electronegativity is not an experimentally pure property, but the result of a calculation that can be performed in different ways). The sulfur derivative would be least reactive.
There are still several different oxygen derivatives to compare: carboxylic acids (OH), carboxylates (O-), esters (OR, in which R is an alkyl or carbon chain) and acid anhydrides (OC=O). The easiest to differentiate is the carboxylate, because of its negative charge. It must be less attractive to a nucleophile than the other oxygen derivatives, because it would offer more repulsion to an incoming lone pair.
However, we can't really predict whether it would be any less reactive than the nitrogen, chlorine or sulfur analogues, because who knows whether the charge or the nature of the atom matters more?
As it happens, the charge probably matters more. We learn that simply by looking at the experimental trend and seeing that the carboxylate is the least reactive of all the carboxyloids.
Turning to the other three oxygen derivatives, it would be difficult to differentiate between the effect of a remote hydrogen atom versus an alkyl chain in the ester versus the carboxylic acid, so we'll say those two are about the same. On the other hand, the additional electron-withdrawing carbonyl group in the acid anhydride probably has a profound effect, so we would expect that compound to attract nucleophiles more strongly.
Of course, the series we have produced above is not the "right answer". It does not match the experimentally observed series of carboxyloid reactivities. Nevertheless, it is very useful in terms of building an understanding of carboxyloids. It tells us that electronegativity may play a role here, but that it can't be the only factor.
Some other factor is putting some of the derivatives out of order. In particular, the acid chloride (C=OCl) and the thioester (C=OSR) do not fit.
Exercise \(\PageIndex{2}\)
Lone pair donation from the atom attached to the carbonyl carbon could also influence carboxyloid reactivity. Explain how this factor would affect electrophilicity at the carbonyl carbon and predict the corresponding trend in reactivity. Compare this trend with the information in Figure \(\PageIndex{1}\).
- Answer
-
Electronegativity is an abvious factor that could influence an atom's ability to π-donate, but we just looked at that factor in the previous section, so let's look at another atomic property instead. Of course, different atoms have different sizes. In particular, if we look at the atoms involved in carboxyloid substituents, we can divide them into 2nd row atoms and 3rd row atoms.
It's actually well-documented that the degree of overlap between two orbitals influences how well they bond together. Since carbon is in the second row, it is about the same size as, and overlaps pretty well with, other second row atoms. Third row atoms are a little too big, on the other hand.
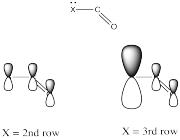
That factor breaks the carboxyloids into two different groups. Assuming π-donation is a major factor, sulfur and chlorine may be placed above the others in tems of reactivity. They cannot donate as well as oxygen or nitrogen can.
From there, differences among the atoms from the same row may be sorted out based on electronegativity differences.
Exercise \(\PageIndex{3}\)
Using the information in Figure \(\PageIndex{1}\), explain why peptides (containing a number of amide bonds, R(C=O)N) are such a common structural feature in biology.
- Answer
-
Amide bonds are among the most stable carboxyloids possible. That stability makes them well-suited to form useful structures that will not decompose easily. Remember, any change that occurs in matter occurs through chemical reactions, including the formation and decomposition of biomaterials. Shutting down a potential chemical reaction means a material will be more durable.
The potential energy curve in Figure \(\PageIndex{1}\) (CX3.1) is a useful index for the interconversion of carboxyloids. In general, it is easy to go downhill on the curve, but more difficult to go uphill. That means that compounds lower down on the ski hill can be made easily from compounds farther up the ski hill.
In general, pi donation from the heteroatom attached to the carbonyl is a primary factor that determines carboxyloid reactivity. The more able the heteroatom is to donate its pi electrons, the less electrophilic is the carbonyl. Nitrogen is very good at donating its lone pair. It is about the same size as the carbon atom it needs to donate to, and it only a little more electronegative than the carbon.
Oxygen (in esters and carboxylic acids) is next in line, since oxygen is more electronegative than nitrogen.
Chlorine and sulfur are a little too large to donate very well to a carbon atom. The size and energy mismatch between these atoms leads to poor pi bonding, and poor pi donation.
Exercise \(\PageIndex{4}\)
Place the following compounds in their relative positions on the ski hill.
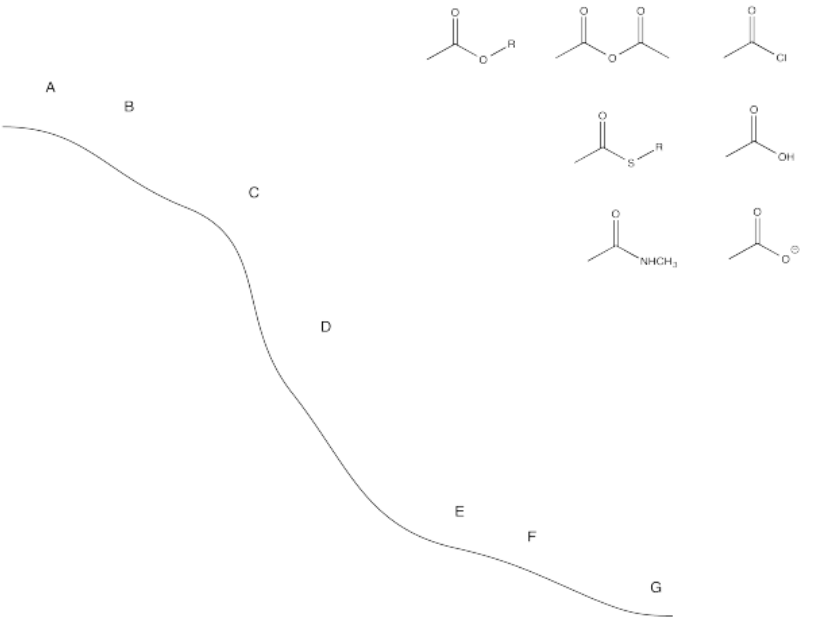
- Answer
-
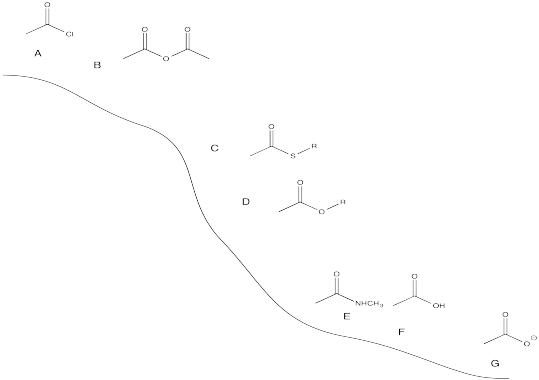
Exercise \(\PageIndex{5}\)
Oxalyl chloride (left) and thionyl chloride (right) are even higher on the ski hill than regular acid chlorides. Propose a reason that explains this relative instability as electrophiles.

- Answer
-
One possibility is the presence of an additional electronegative substituent. In the oxalyl chloride, the presence of an additional carbonyl next to the electrophilic acid chloride group would make each carbonyl even more electrophilic. In the thionyl chloride, the presence of two chlorines, instead of just one, could make this compound much less stable and more electrophilic.


