19.2: Describing Electrochemical Cells
- Page ID
- 349769
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To distinguish between galvanic and electrolytic cells.
In any electrochemical process, electrons flow from one chemical substance to another, driven by an oxidation–reduction (redox) reaction. As we described in Chapter 7, a redox reaction occurs when electrons are transferred from a substance that is oxidized to one that is being reduced. The reductant (a substance that is capable of donating electrons and in the process is oxidized) is the substance that loses electrons and is oxidized in the process; the oxidant (A substance that is capable of accepting electrons and in the process is reduced) is the species that gains electrons and is reduced in the process. The associated potential energy is determined by the potential difference between the valence electrons in atoms of different elements.
Because it is impossible to have a reduction without an oxidation and vice versa, a redox reaction can be described as two half-reactions (reactions that represent either the oxidation half or the reduction half of an oxidation–reduction (redox) reaction), one representing the oxidation process and one the reduction process. For the reaction of zinc with bromine, the overall chemical reaction is as follows:
\[ Zn\left ( s \right ) + Br_{2}\left ( l \right ) \rightarrow Zn^{2+}\left ( aq \right ) + 2Br^{-}\left ( aq \right ) \tag{19.1.1} \]
The half-reactions are as follows:
\[ reduction\;half\;reaction: Br_{2}\left ( l \right )+2e^{-} \rightarrow 2Br^{-}\left ( aq \right ) \tag{19.1.2} \]
\[ oxidation\;half\;reaction: Zn\left ( s \right ) \rightarrow Zn^{2+}\left ( aq \right ) +2e^{-} \tag{19.1.3} \]
Each half-reaction is written to show what is actually occurring in the system; Zn is the reductant in this reaction (it loses electrons), and Br2 is the oxidant (it gains electrons). Adding the two half-reactions gives the overall chemical reaction (Equation 19.1). A redox reaction is balanced when the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Like any balanced chemical equation, the overall process is electrically neutral; that is, the net charge is the same on both sides of the equation.
Note the Pattern
In any redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant.
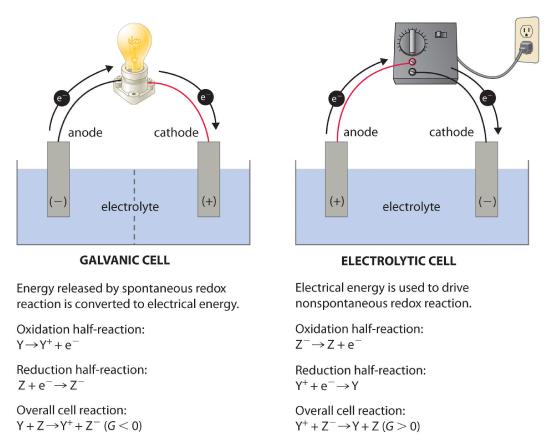
In most of our discussions of chemical reactions, we have assumed that the reactants are in intimate physical contact with one another. Acid–base reactions, for example, are usually carried out with the acid and the base dispersed in a single phase, such as a liquid solution. With redox reactions, however, it is possible to physically separate the oxidation and reduction half-reactions in space, as long as there is a complete circuit, including an external electrical connection, such as a wire, between the two half-reactions. As the reaction progresses, the electrons flow from the reductant to the oxidant over this electrical connection, producing an electric current that can be used to do work. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell (an apparatus that generates electricity from a spontaneous oxidation–reduction (redox) reaction or, conversely, uses electricity to drive a nonspontaneous redox reaction).
There are two types of electrochemical cells: galvanic cells and electrolytic cells. A galvanic (voltaic) cell (an electrochemical cell that uses the energy released during a spontaneous oxidation–reduction (redox) reaction (ΔGo) to generate electricity). Galvanic cells are named for the Italian physicist and physician Luigi Galvani (1737–1798), who observed that dissected frog leg muscles twitched when a small electric shock was applied, demonstrating the electrical nature of nerve impulses. uses the energy released during a spontaneous redox reaction (ΔG < 0) to generate electricity. This type of electrochemical cell is often called a voltaic cell after its inventor, the Italian physicist Alessandro Volta (1745–1827). In contrast, an electrolytic cellAn electrochemical cell that consumes electrical energy from an external source to drive a nonspontaneous (ΔGo) oxidation–reduction (redox) reaction. consumes electrical energy from an external source, using it to cause a nonspontaneous redox reaction to occur (ΔG > 0). Both types contain two electrodesA solid metal connected by an electrolyte and an external circuit that provides an electrical connection between systems in an electrochemical cell (galvanic or electrolytic)., which are solid metals connected to an external circuit that provides an electrical connection between the two parts of the system (Figure 19.1.1). The oxidation half-reaction occurs at one electrode (the anodeOne of two electrodes in an electrochemical cell, it is the site of the oxidation half-reaction.), and the reduction half-reaction occurs at the other (the cathodeOne of two electrodes in an electrochemical cell, it is the site of the reduction half-reaction.). When the circuit is closed, electrons flow from the anode to the cathode. The electrodes are also connected by an electrolyte, an ionic substance or solution that allows ions to transfer between the electrode compartments, thereby maintaining the system’s electrical neutrality. In this section, we focus on reactions that occur in galvanic cells. We discuss electrolytic cells in Section 19.7.
Galvanic (Voltaic) Cells
To illustrate the basic principles of a galvanic cell, let’s consider the reaction of metallic zinc with cupric ion (Cu2+) to give copper metal and Zn2+ ion. The balanced chemical equation is as follows:
\( Zn\left ( s \right ) + Cu^{2+}\left (aq \right ) \rightarrow Zn^{2+} \left (aq \right ) + Cu \left ( s \right ) \tag{19.1.4} \)
We can cause this reaction to occur by inserting zinc powder into an aqueous solution of copper(II) sulfate. As the reaction proceeds, the zinc dissolves, and a mass of metallic copper forms (Figure 19.1.2). These changes occur spontaneously, but all the energy released is in the form of heat rather than in a form that can be used to do work.
Figure 19.1.2 The Reaction of Metallic Zinc with Aqueous Copper(II) Ions in a Single Compartment When zinc powder is inserted into a beaker that contains an aqueous solution of copper(II) sulfate, a spontaneous redox reaction occurs: the zinc electrode dissolves to give Zn2+(aq) ions, while Cu2+(aq) ions are simultaneously reduced to metallic copper. The reaction occurs so rapidly that the copper is deposited as very fine particles that appear black, rather than the usual reddish color of copper. The reaction demonstration starts at 3:00 in the video, the chemistry of the reaction is discussed in the first three minutes and at the end
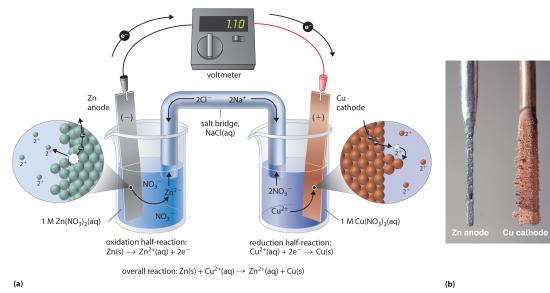
This same reaction can be carried out using the galvanic cell illustrated in part (a) in Figure 19.1.3. To assemble the cell, a copper strip is inserted into a beaker that contains a 1 M solution of Cu2+ ions, and a zinc strip is inserted into a different beaker that contains a 1 M solution of Zn2+ ions. The two metal strips, which serve as electrodes, are connected by a wire, and the compartments are connected by a salt bridge (a U-shaped tube inserted into both solutions of a galvanic cell that contains a concentrated liquid or gelled electrolyte and completes the circuit between the anode and the cathode), a U-shaped tube inserted into both solutions that contains a concentrated liquid or gelled electrolyte. The ions in the salt bridge are selected so that they do not interfere with the electrochemical reaction by being oxidized or reduced themselves or by forming a precipitate or complex; commonly used cations and anions are Na+ or K+ and NO3− or SO42−, respectively. (The ions in the salt bridge do not have to be the same as those in the redox couple in either compartment.) When the circuit is closed, a spontaneous reaction occurs: zinc metal is oxidized to Zn2+ ions at the zinc electrode (the anode), and Cu2+ ions are reduced to Cu metal at the copper electrode (the cathode). As the reaction progresses, the zinc strip dissolves, and the concentration of Zn2+ ions in the Zn2+ solution increases; simultaneously, the copper strip gains mass, and the concentration of Cu2+ ions in the Cu2+ solution decreases (part (b) in Figure 19.1.3). Thus we have carried out the same reaction as we did using a single beaker, but this time the oxidative and reductive half-reactions are physically separated from each other. The electrons that are released at the anode flow through the wire, producing an electric current. Galvanic cells therefore transform chemical energy into electrical energy that can then be used to do work.
The electrolyte in the salt bridge serves two purposes: it completes the circuit by carrying electrical charge and maintains electrical neutrality in both solutions by allowing ions to migrate between them. The identity of the salt in a salt bridge is unimportant, as long as the component ions do not react or undergo a redox reaction under the operating conditions of the cell. Without such a connection, the total positive charge in the Zn2+ solution would increase as the zinc metal dissolves, and the total positive charge in the Cu2+ solution would decrease. The salt bridge allows charges to be neutralized by a flow of anions into the Zn2+ solution and a flow of cations into the Cu2+ solution. In the absence of a salt bridge or some other similar connection, the reaction would rapidly cease because electrical neutrality could not be maintained.
A galvanic cell. This galvanic cell illustrates the use of a salt bridge to connect two solutions generating a voltage.
A voltmeter can be used to measure the difference in electrical potential between the two compartments. Opening the switch that connects the wires to the anode and the cathode prevents a current from flowing, so no chemical reaction occurs. With the switch closed, however, the external circuit is closed, and an electric current can flow from the anode to the cathode. The potential (Ecell)Related to the energy needed to move a charged particle in an electric field, it is the difference in electrical potential beween two half-reactions. of the cell, measured in volts, is the difference in electrical potential between the two half-reactions and is related to the energy needed to move a charged particle in an electric field. In the cell we have described, the voltmeter indicates a potential of 1.10 V (part (a) in Figure 19.1.3 ). Because electrons from the oxidation half-reaction are released at the anode, the anode in a galvanic cell is negatively charged. The cathode, which attracts electrons, is positively charged.
Not all electrodes undergo a chemical transformation during a redox reaction. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the overall electrochemical reaction. This phenomenon is illustrated in Example 1.
Note the Pattern
A galvanic (voltaic) cell converts the energy released by a spontaneous chemical reaction to electrical energy. An electrolytic cell consumes electrical energy from an external source to drive a nonspontaneous chemical reaction.
Example 19.1.1
A chemist has constructed a galvanic cell consisting of two beakers. One beaker contains a strip of tin immersed in aqueous sulfuric acid, and the other contains a platinum electrode immersed in aqueous nitric acid. The two solutions are connected by a salt bridge, and the electrodes are connected by a wire. Current begins to flow, and bubbles of a gas appear at the platinum electrode. The spontaneous redox reaction that occurs is described by the following balanced chemical equation:
3Sn(s) + 2NO3−(aq) + 8H+(aq) → 3Sn2+(aq) + 2NO(g) + 4H2O(l)
For this galvanic cell,
- write the half-reaction that occurs at each electrode.
- indicate which electrode is the cathode and which is the anode.
- indicate which electrode is the positive electrode and which is the negative electrode.
Given: galvanic cell and redox reaction
Asked for: half-reactions, identity of anode and cathode, and electrode assignment as positive or negative
Strategy:
A Identify the oxidation half-reaction and the reduction half-reaction. Then identify the anode and cathode from the half-reaction that occurs at each electrode.
B From the direction of electron flow, assign each electrode as either positive or negative.
Solution:
- A In the reduction half-reaction, nitrate is reduced to nitric oxide. (The nitric oxide would then react with oxygen in the air to form NO2, with its characteristic red-brown color.) In the oxidation half-reaction, metallic tin is oxidized. The half-reactions corresponding to the actual reactions that occur in the system are as follows:
reduction: NO3−(aq) + 4H+(aq) + 3e− → NO(g) + 2H2O(l) oxidation: Sn(s) → Sn2+(aq) + 2e−
Thus nitrate is reduced to NO, while the tin electrode is oxidized to Sn2+.
- Because the reduction reaction occurs at the Pt electrode, it is the cathode. Conversely, the oxidation reaction occurs at the tin electrode, so it is the anode.
- B Electrons flow from the tin electrode through the wire to the platinum electrode, where they transfer to nitrate. The electric circuit is completed by the salt bridge, which permits the diffusion of cations toward the cathode and anions toward the anode. Because electrons flow from the tin electrode, it must be electrically negative. In contrast, electrons flow toward the Pt electrode, so that electrode must be electrically positive.
Exercise
Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. One beaker contains a solution of MnO4− in dilute sulfuric acid and has a Pt electrode. The other beaker contains a solution of Sn2+ in dilute sulfuric acid, also with a Pt electrode. When the two electrodes are connected by a wire, current flows and a spontaneous reaction occurs that is described by the following balanced chemical equation:
2MnO4−(aq) + 5Sn2+(aq) + 16H+(aq) → 2Mn2+(aq) + 5Sn4+(aq) + 8H2O(l)
For this galvanic cell,
- write the half-reaction that occurs at each electrode.
- indicate which electrode is the cathode and which is the anode.
- indicate which electrode is positive and which is negative.
Answer
- MnO4−(aq) + 8H+(aq) + 5e− → Mn2+(aq) + 4H2O(l); Sn2+(aq) → Sn4+(aq) + 2e−
- The Pt electrode in the permanganate solution is the cathode; the one in the tin solution is the anode.
- The cathode (electrode in beaker that contains the permanganate solution) is positive, and the anode (electrode in beaker that contains the tin solution) is negative.
Constructing a Cell Diagram
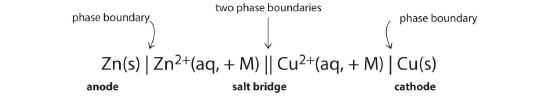
Because it is somewhat cumbersome to describe any given galvanic cell in words, a more convenient notation has been developed. In this line notation, called a cell diagram, the identity of the electrodes and the chemical contents of the compartments are indicated by their chemical formulas, with the anode written on the far left and the cathode on the far right. Phase boundaries are shown by single vertical lines, and the salt bridge, which has two phase boundaries, by a double vertical line. Thus the cell diagram for the Zn/Cu cell shown in part (a) in Figure 19.1.3 is written as follows:
Figure 19.1.4 A cell diagram includes solution concentrations when they are provided.
Galvanic cells can have arrangements other than the examples we have seen so far. For example, the voltage produced by a redox reaction can be measured more accurately using two electrodes immersed in a single beaker containing an electrolyte that completes the circuit. This arrangement reduces errors caused by resistance to the flow of charge at a boundary, called the junction potential. One example of this type of galvanic cell is as follows:
\( Pt\left (s \right )\mid H_{2}\left (g \right )\mid HCl\left (aq \right )\mid AgCl\left (s \right )\mid Ag\left (s \right ) \tag{19.1.5} \)
This cell diagram does not include a double vertical line representing a salt bridge because there is no salt bridge providing a junction between two dissimilar solutions. Moreover, solution concentrations have not been specified, so they are not included in the cell diagram. The half-reactions and the overall reaction for this cell are as follows:
\( cathode\;reaction: AgCl\left (s \right ) + e^{-} \rightarrow Ag \left (s \right ) + Cl^{-}\left (aq \right ) \tag{19.1.6} \)
\( anode\;reaction: \dfrac{1}{2}H_{2}\left (g \right ) \rightarrow H^{+} \left (aq \right ) + e^{-} \tag{19.1.7} \)
\( overall: AgCl\left (s \right ) +\dfrac{1}{2}H_{2}\left (g \right ) \rightarrow Ag \left (s \right ) + Cl^{-}\left (aq \right ) + H^{+} \left (aq \right ) \tag{19.1.8} \)
A single-compartment galvanic cell will initially exhibit the same voltage as a galvanic cell constructed using separate compartments, but it will discharge rapidly because of the direct reaction of the reactant at the anode with the oxidized member of the cathodic redox couple. Consequently, cells of this type are not particularly useful for producing electricity.
Example 19.1.2
Draw a cell diagram for the galvanic cell described in Example 1. The balanced chemical reaction is as follows:
3Sn(s) + 2NO3−(aq) + 8H+(aq) → 3Sn2+(aq) + 2NO(g) + 4H2O(l)
Given: galvanic cell and redox reaction
Asked for: cell diagram
Strategy:
Using the symbols described, write the cell diagram beginning with the oxidation half-reaction on the left.
Solution:
The anode is the tin strip, and the cathode is the Pt electrode. Beginning on the left with the anode, we indicate the phase boundary between the electrode and the tin solution by a vertical bar. The anode compartment is thus Sn(s)∣Sn2+(aq). We could include H2SO4(aq) with the contents of the anode compartment, but the sulfate ion (as HSO4−) does not participate in the overall reaction, so it does not need to be specifically indicated. The cathode compartment contains aqueous nitric acid, which does participate in the overall reaction, together with the product of the reaction (NO) and the Pt electrode. These are written as HNO3(aq)∣NO(g)∣Pt(s), with single vertical bars indicating the phase boundaries. Combining the two compartments and using a double vertical bar to indicate the salt bridge,
Sn(s)∣Sn2+(aq)∥HNO3(aq)∣NO(g)∣Pt(s)
The solution concentrations were not specified, so they are not included in this cell diagram.
Exercise
Draw a cell diagram for the following reaction, assuming the concentration of Ag+ and Mg2+ are each 1 M:
Mg(s) + 2Ag+(aq) → Mg2+(aq) + 2Ag(s)
Answer: Mg(s)∣Mg2+(aq, 1 M)∥Ag+(aq, 1 M)∣Ag(s)
Summary
Electrochemistry is the study of the relationship between electricity and chemical reactions. The oxidation–reduction reaction that occurs during an electrochemical process consists of two half-reactions, one representing the oxidation process and one the reduction process. The sum of the half-reactions gives the overall chemical reaction. The overall redox reaction is balanced when the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. An electric current is produced from the flow of electrons from the reductant to the oxidant. An electrochemical cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is consumed to drive a nonspontaneous redox reaction. Both types of cells use two electrodes that provide an electrical connection between systems that are separated in space. The oxidative half-reaction occurs at the anode, and the reductive half-reaction occurs at the cathode. A salt bridge connects the separated solutions, allowing ions to migrate to either solution to ensure the system’s electrical neutrality. A voltmeter is a device that measures the flow of electric current between two half-reactions. The potential of a cell, measured in volts, is the energy needed to move a charged particle in an electric field. An electrochemical cell can be described using line notation called a cell diagram, in which vertical lines indicate phase boundaries and the location of the salt bridge. Resistance to the flow of charge at a boundary is called the junction potential.
Key Takeaway
- A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur.
Conceptual Problems
-
Is 2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(l) an oxidation–reduction reaction? Why or why not?
-
If two half-reactions are physically separated, how is it possible for a redox reaction to occur? What is the name of the apparatus in which two half-reactions are carried out simultaneously?
-
What is the difference between a galvanic cell and an electrolytic cell? Which would you use to generate electricity?
-
What is the purpose of a salt bridge in a galvanic cell? Is it always necessary to use a salt bridge in a galvanic cell?
-
One criterion for a good salt bridge is that it contains ions that have similar rates of diffusion in aqueous solution, as K+ and Cl− ions do. What would happen if the diffusion rates of the anions and cations differed significantly?
-
It is often more accurate to measure the potential of a redox reaction by immersing two electrodes in a single beaker rather than in two beakers. Why?
Answer
-
A large difference in cation/anion diffusion rates would increase resistance in the salt bridge and limit electron flow through the circuit.
Numerical Problems
-
Copper(I) sulfate forms a bright blue solution in water. If a piece of zinc metal is placed in a beaker of aqueous CuSO4 solution, the blue color fades with time, the zinc strip begins to erode, and a black solid forms around the zinc strip. What is happening? Write half-reactions to show the chemical changes that are occurring. What will happen if a piece of copper metal is placed in a colorless aqueous solution of ZnCl2?
-
Consider the following spontaneous redox reaction: NO3−(aq) + H+(aq) + SO32−(aq) → SO42−(aq) + HNO2(aq).
- Write the two half-reactions for this overall reaction.
- If the reaction is carried out in a galvanic cell using an inert electrode in each compartment, which electrode corresponds to which half-reaction?
- Which electrode is negatively charged, and which is positively charged?
-
The reaction Pb(s) + 2VO2+(aq) + 4H+(aq) → Pb2+(aq) + 2V3+(aq) + 2H2O(l) occurs spontaneously.
- Write the two half-reactions for this redox reaction.
- If the reaction is carried out in a galvanic cell using an inert electrode in each compartment, which reaction occurs at the cathode and which occurs at the anode?
- Which electrode is positively charged, and which is negatively charged?
-
Phenolphthalein is an indicator that turns pink under basic conditions. When an iron nail is placed in a gel that contains [Fe(CN)6]3−, the gel around the nail begins to turn pink. What is occurring? Write the half-reactions and then write the overall redox reaction.
-
Sulfate is reduced to HS− in the presence of glucose, which is oxidized to bicarbonate. Write the two half-reactions corresponding to this process. What is the equation for the overall reaction?
-
Write the spontaneous half-reactions and the overall reaction for each proposed cell diagram. State which half-reaction occurs at the anode and which occurs at the cathode.
- Pb(s)∣PbSO4(s)∣SO42−(aq)∥Cu2+(aq)∣Cu(s)
- Hg(l)∣Hg2Cl2(s)∣Cl−(aq) ∥ Cd2+(aq)∣Cd(s)
-
For each galvanic cell represented by these cell diagrams, determine the spontaneous half-reactions and the overall reaction. Indicate which reaction occurs at the anode and which occurs at the cathode.
- Zn(s)∣Zn2+(aq) ∥ H+(aq)∣H2(g), Pt(s)
- Ag(s)∣AgCl(s)∣Cl−(aq) ∥ H+(aq)∣H2(g)∣Pt(s)
- Pt(s)∣H2(g)∣H+(aq) ∥ Fe2+(aq), Fe3+(aq)∣Pt(s)
-
For each redox reaction, write the half-reactions and draw the cell diagram for a galvanic cell in which the overall reaction occurs spontaneously. Identify each electrode as either positive or negative.
- Ag(s) + Fe3+(aq) → Ag+(aq) + Fe2+(aq)
- Fe3+(aq) + 1/2H2(g) → Fe2+(aq) + H+(aq)
-
Write the half-reactions for each overall reaction, decide whether the reaction will occur spontaneously, and construct a cell diagram for a galvanic cell in which a spontaneous reaction will occur.
- 2Cl−(aq) + Br2(l) → Cl2(g) + 2Br−(aq)
- 2NO2(g) + 2OH−(aq) → NO2−(aq) + NO3−(aq) + H2O(l)
- 2H2O(l) + 2Cl−(aq) → H2(g) + Cl2(g) + 2OH−(aq)
- C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
-
Write the half-reactions for each overall reaction, decide whether the reaction will occur spontaneously, and construct a cell diagram for a galvanic cell in which a spontaneous reaction will occur.
- Co(s) + Fe2+(aq) → Co2+(aq) + Fe(s)
- O2(g) + 4H+(aq) + 4Fe2+(aq) → 2H2O(l) + 4Fe3+(aq)
- 6Hg2+(aq) + 2NO3−(aq) + 8H+ → 3Hg22+(aq) + 2NO(g) + 4H2O(l)
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
Answers
-
reduction: SO42−(aq) + 9H+(aq) + 8e− → HS−(aq) + 4H2O(l) oxidation: C6H12O6(aq) + 12H2O(l) → 6HCO3−(g) + 30H+(aq) + 24e− overall: C6H12O6(aq) + 3SO42−(aq) → 6HCO3−(g) + 3H+(aq) + 3HS−(aq)
-
- reduction: 2H+(aq) + 2e− → H2(aq); cathode;
oxidation: Zn(s) → Zn2+(aq) + 2e−; anode;
overall: Zn(s) + 2H+(aq) → Zn2+(aq) + H2(aq)
- reduction: AgCl(s) + e− → Ag(s) + Cl−(aq); cathode;
oxidation: H2(g) → 2H+(aq) + 2e−; anode;
overall: AgCl(s) + H2(g) → 2H+(aq) + Ag(s) + Cl−(aq)
- reduction: Fe3+(aq) + e− → Fe2+(aq); cathode;
oxidation: H2(g) → 2H+(aq) + 2e−; anode;
overall: 2Fe3+(aq) + H2(g) → 2H+(aq) + 2Fe2+(aq)
- reduction: 2H+(aq) + 2e− → H2(aq); cathode;