4.6: Degradation
- Page ID
- 294550
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)3.7. Degradation
3.7.1. Chemical and photochemical degradation processes
Authors: John Parsons
Reviewers: Steven Droge, Kristopher McNeill
Leaning objectives:
You should be able to:
- understand the role of chemical and photochemical reactions in the removal of organic chemicals from the environment
- understand the most important chemical and photochemical reactions in the environment
- understand the role of direct and indirect photodegradation
Keywords: Environmental degradation reactions, Hydrolysis, Reduction, Dehalogenation, Oxidation, Photodegradation
Introduction
Transformation of organic chemicals in the environment can occur by a variety of reactions. These may be purely chemical reactions, such as hydrolyses or redox reactions, photochemical reactions with the direct or indirect involvement of light, or biochemical reactions. Such transformations can change the biological activity (toxicity) of a molecule; it can change the physico-chemical properties and thus change its environmental partitioning processes; it can change its bioavailability, for example facilitating biodegradation; or it may contribute to the complete removal (mineralization) of the chemical from the environment. In many cases, chemicals may be removed by combinations of these different processes and it is sometimes difficult to unequivocally identify the contributions of the different mechanisms. Indeed, combinations of different mechanisms are sometimes important, for example in cases where microbial activity is responsible for creating conditions that favour chemical reactions. Here we will focus on two types of reactions: Abiotic (dark) reactions and photochemical reactions. Biodegradation reactions are covered elsewhere (see section on Biodegradation).
Chemical degradation
Hydrolytic reactions are important chemical reactions removing organic contaminants and are particularly important for chemicals containing acid derivatives as functional groups. Common examples of such chemicals are pesticides of the organophosphate and carbamate classes such as parathion, diazinon, aldicarb and carbaryl. Organophosphate chemicals are also used as flame retardants and are widely distributed in the environment. Some examples of hydrolysis reactions are shown in Figure 1.
As the name suggests, hydrolysis reactions involve using water (hydro-) to break (-lysis) a bond. Hydrolyses are reactions with water to produce an acid and either an alcohol or amine as products. Hydrolyses can be catalysed by either OH- or H+ ions and their rates are therefore pH dependent. Some examples of pH-dependent ester hydrolysis reactions are shown in Figure 2.
Halogenated organic molecules may also be hydrolysed to form alcohols (releasing the halogen as a halide ion). The rates of these reactions vary strongly depending on the structure of the organohalogen molecule and the halogen substituent (with Br and I being substituted more rapidly than Cl, and much more rapidly than F) and in general the rates of these reactions are too slow to be of more than minor importance except for tertiary organohalogens and secondary organohalogens with Br and I (Schwarzenbach et al. 2017).

In some cases, other substitution reactions not involving water as reactant may be important. Some examples include Cl- in seawater converting CH3I to CH3Cl and reaction of thiols with alkyl bromines in anaerobic groundwater and sediment porewater under sulfate-reducing conditions (Schwarzenbach et al. 2017)
Redox (reduction and oxidation) reactions are another important reaction class involved in the degradation of organic chemicals. In the presence of oxygen, the oxidation of organic chemicals is thermodynamically favourable but occurs at insignificant rates unless oxygen is activated in the form of oxygen radicals or peroxides (following light absorption for example, see below) or if the reaction is catalysed by transition metals or transition metal-containing enzymes (see the sections on Biodegradation and Xenobiotic metabolism and defence).
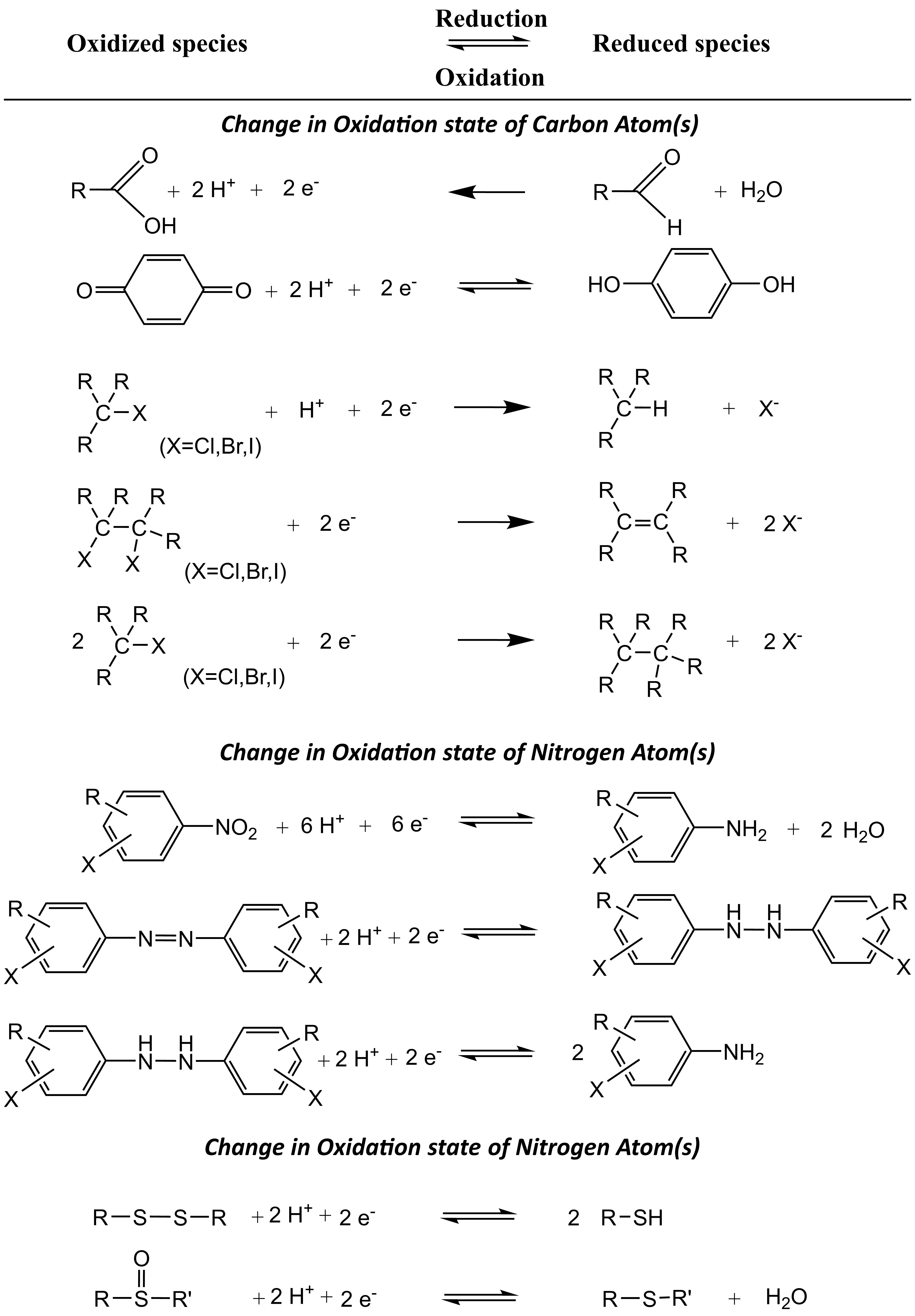
Reduction reactions are important redox reactions for environmental contaminants in anaerobic environments such as sediment and groundwater aquifers. Under these conditions, organic chemicals containing reducible functional groups such as carboxylic acids and nitro groups undergo reduction reactions (Table 1).
Table 1: Examples of chemical redox reactions that may occur in the environment (adapted from Schwarzenbach et al. 2017)

Photodegradation
Sunlight is an important source of energy to initiate chemical reactions and photochemical reactions are particularly important in the atmosphere. Aromatic compounds and other chemicals containing unsaturated bonds that are able to absorb light in the frequency range available in sunlight become exited (energized) and this can lead to chemical reactions. These reactions lead to cleavage of bonds between carbon atoms and other atoms such as halogens to produce radical species. These radicals are highly reactive and react further to remove hydrogen or OH radicals from water to produce C-H or C-OH bonds or may react with themselves to produce larger molecules. Well known examples of atmospheric photochemical stratospheric reactions of CFCs that have had a negative impact on the so-called ozone layer and photochemical oxidations of hydrocarbons that are involved in the generation of smog.
In the aquatic environment, light penetration is sufficient to lead to photochemical reactions of organic chemicals at the water surface or in the top layer of clear water. The presence of particles in a waterbody reduces light intensity through light scattering as does dissolved organic matter through light absorption. Photodegradation contributes significantly to removing oil spills and appears to favour the degradation of longer chain alkanes compared to the preferential attack of linear and small alkanes by biodegradation (Garrett et al., 1998). Cycloalkanes and aromatic hydrocarbons are also removed by photodegradation (D'Auria et al., 2009). There is comparatively little known about the role photodegradation of other organic pollutants in the marine environment although there is, for example, evidence that triclosan is removed by photolysis in the German Bight area of the North Sea (Xie et al., 2008). In the soil environment, there is some evidence that photodegradation may contribute to the removal of a variety of organic chemicals such as pesticides and chemicals present in sewage sludge that is used as a soil amendment but the significance of this process is unclear. Similarly, chemicals that have accumulated in ice, for example as a result of long range transport to polar regions, also seem to be susceptible to photodegradation. Some examples of photodegradation reactions are shown in Figure 4.

An important category of photochemical reactions are indirect reactions in which organic chemicals react with photochemically produced radicals, in particular with reactive oxygen species such as OH radicals, ozone and singlet oxygen. These reactive species are present at very low concentrations but are so reactive that under certain conditions they can contribute significantly to the removal of organic chemicals. Products of these reactions are a variety of oxidized derivatives which are themselves radicals and therefore react further. OH radicals are the most important of these photochemically produced species and can react with organic chemicals by removing hydrogen radicals, reacting with unsaturated bonds in alkenes, aromatics etc. to produce hydroxylated products. In water, natural organic matter absorbs light and can participate in indirect photodegradation reactions. Other constituents in surface water, such as nitrogen oxides and iron complexes may also be involved in indirect photodegradation reactions.
References
Schwarzenbach, R.P., Gschwend, P.M., Imboden, D.M. (2017). Environmental Organic Chemistry, Third Edition, Wiley, ISBN 978-1-118-76723-8
van Leeuwen, C.J., Vermeire, T.G. (2007). Risk Assessment of Chemicals: An Introduction (2nd ed.), Springer, ISBN 978-1-4020-6101-1
Which organic chemicals would you expect to undergo hydrolytic degradation in the environment? Explain why these reaction depend on the pH.
Reductive dehalogenation reactions are often observed to occur for organochlorine compounds in anaerobic environments. Why are these reactions called reductive dehalogenation? What products would you expect to be formed by the reductive dehalogenation of tetrachloroethene (Cl2C=CCl2)? Describe two ways in which bacteria are involved in these reactions.
In which environmental compartments is photochemical transformation or photodegradation a potentially important degradation mechanism for organic chemicals and why is this the case? Explain with examples the differences between direct and indirect photodegradation in the environment.
3.7.2. Biodegradation
Author: John Parsons
Reviewers: Steven Droge, Russell Davenport
Leaning objectives:
You should be able to:
- the contribution of biochemical reactions in removing chemicals from the environment
- explain the differences between biotransformation, primary biodegradation and mineralization
- describe the most important biodegradation reactions under aerobic and anaerobic conditions
Keywords: Primary biodegradation, mineralisation, readily biodegradable chemicals, persistent chemicals, oxygenation reactions, reductions reactions
Introduction:
Biodegradation and biotransformation both refer to degradation reactions that are catalyzed by enzymes. In general, biodegradation is usually used to describe the degradation carried out by microorganisms and biotransformation often refers to reactions that follow the uptake of chemicals by higher organisms. This distinction is important and arises from the role that bacteria and other microorganisms play in natural biogeochemical cycles. As a result, microorganisms have the capacity to degrade most (perhaps all) naturally occurring organic chemicals in organic matter and convert them to inorganic end products. These reactions supply the microorganisms with the nutrients and energy they need to grow. This broad degradative capacity means that they are able to degrade many anthropogenic chemicals and potentially convert them to inorganic end products, a process referred to as mineralisation.
Although higher organisms are also able to degrade (metabolise) many anthropogenic chemicals, these chemicals are not taken up as source of nutrients and energy. Many anthropogenic chemicals can disturb cell functioning processes, and the biotransformation process has been proposed as a detoxification mechanism. Undesirable chemicals that may accumulate to potentially harmful levels are converted to products that are more rapidly excreted. In most cases, a polar and/or ionizable unit is attached to the chemical in one or two steps, making the compound more soluble in blood and more readily removed via the kidneys to the urine. This also renders most hazardous chemicals less toxic than the original chemical. Such biotransformation steps always costs energy (ATP, or through the use of e.g. NADH or NADPH in the enzymatic reactions) from the organism. Biotransformation is sometimes also used to describe degradation by microorganisms when this is limited to a conversion of a chemical into a new product.
Biodegradation is for many organic contaminants the major process that removes them from the environment. Measuring the rates of biodegradation therefore is a prominent aspect of chemical risk assessment. Internationally recognized standardised protocols have been developed to measure biodegradation rates of chemicals. Well know examples of these are the OCED Guidelines. These guidelines include screening tests designed to identify chemicals can be regarded as readily (i.e. rapidly) biodegradable as well as more complex tests to measure biodegradation rates of chemicals that degrade slowly in a variety of simulated environments. For more complex mechanistic studies, microorganisms able to degrade specific chemicals are isolated from environmental samples and cultivated in laboratory systems.
In principle, biodegradation of a chemical can be determined by either following the concentration of the chemical during the test or by following the conversion to end products (in most cases by either measuring oxygen consumption or CO2 production). Although measuring the concentration gives the most directly relevant information on a chemical, it requires the availability or development of analytical methods which is not always within the capability of routine testing laboratories. Measuring the conversion to CO2 is comparatively straightforward but the production of CO2 from other chemicals present in the test system (such as soil or dissolved organic matter) should be accounted for. This can be done by using 14C-labelled chemicals in the tests but not all laboratories have facilities for this. The main advantage of this approach is that demonstration of quantitative conversion of a chemical to CO2 etc. means that there is no concern about the accumulation of potentially toxic metabolites.
Since it is an enzymatically catalysed process, the rates of biodegradation should be modelled using the Michaelis Menten kinetics, or Monod kinetics if growth of the microorganisms is taken into account. In practice, however, first order kinetics are often used to model biodegradation in the absence of significant growth of the degrading microorganisms. This is more convenient that using Michaelis Menten kinetics but there is some justification for this simplification since the concentrations of chemicals in the environment are in general much lower than the half saturation concentrations of the degrading enzymes.
Table 1. Influence of molecular structure on the biodegradability of chemicals in the aerobic environment.
|
Type of compounds or substituents |
More biodegradable |
Less biodegradable |
|
hydrocarbons |
linear alkanes < C12 |
linear alkanes > C12 |
|
alkanes with not too high molecular weight |
high molecular weight alkanes |
|
|
linear chain |
branched chain |
|
|
-C-C-C- |
-C-O-C- |
|
|
aliphatic |
aromatic |
|
|
aliphatic chlorine |
Cl more than 6 carbons from terminal C |
Cl at less than 6 carbons from terminal C |
|
Substituents to an aromatic ring |
-OH |
-F |
|
-CO2H |
-Cl |
|
|
-NH2 |
-NO2 |
|
|
-OCH3 |
-CF3 |
Whether expressed as terms of first order kinetics or Michaelis Menten parameters, rates of biodegradation vary widely for different chemicals showing that chemical structure has a large impact on biodegradation. Large variations in biodegradation rates are however often observed for the same chemical in different experimental systems. This shows that environmental properties and conditions also play a key role in determining removal by biodegradation and it is often almost impossible to distinguish the effects of chemical properties from those of environmental properties. In other words, there is no such thing as an intrinsic biodegradation rate of a chemical. Nevertheless, we can derive some generic relationships between the structure and biodegradability of chemicals, as listed in Table 1. Examples are that branched hydrocarbon structures are degraded more slowly than linear hydrocarbon structures, and cyclic and in particular aromatic chemicals are degraded more slowly than aliphatic (non-aromatic) chemicals. Substituents and functional groups also have a major impact on biodegradability with halogens and other electron withdrawing substituents having strongly negative effects. It is therefore no surprise than the list of persistent organic pollutants is dominated by organohalogen compounds and in particular those with aromatic or alicyclic structures.
It should be recognized that biodegradation rates have often been observed to change over time. Long term exposure of microbial communities to new chemicals has often been observed to lead to increasing biodegradation rates. This phenomenon is called adaptation or acclimation and is often the case following repeated application of a pesticide at the same location. An example is shown for atrazine in Figure 2 where degradation rates increase following longer exposure to the pesticide.


Another recent example is the differences in biodegradation rates of the builder L-GLDA (tetrasodium glutamate diacetate) by activated sludge from different waste water treatment plants in the USA. Sludge from regions where L-GLDA was not or only recently on the market required long lag time before degradation started whereas sludge from regions where L-GLDA -containing products had been available for several months required shorted lag phases.

Adaptation can results from i) shifts in composition or abundances of species in a bacterial community, ii) mutations within single populations, iii) horizontal transfer of DNA or iv) genetic recombination events, or combinations of these.
Biodegradation reactions and pathways
Biodegradation of chemicals that we regard as pollutants takes place when these chemicals are incorporated into the metabolism of microorganisms. The reactions involved in biodegradation are therefore similar to those involved in common metabolic reactions, such as hydrolyses, oxidations and reductions. Since the conversion of an organic chemical to CO2 is an overall oxidation reaction, oxidation reactions involving molecular oxygen are probably the most important reactions. These reactions with oxygen are often the first but essential step in degradation and can be regarded as activation step converting relatively stable molecules to more reactive intermediates. This is particularly important for aromatic chemicals since oxygenation is required to make aromatic rings susceptible to ring cleavage and further degradation. These reactions are catalysed by enzymes called oxygenases of which there are broadly speaking two classes. Monoxygenases are enzymes catalysing reactions in which one oxygen atom of O2 reacts with an organic molecule to produce a hydroxylated product. Examples of such enzymes are the cytochrome P450 family and are present in all organisms. These enzymes are for example involved in the oxidation of alkanes to carboxylic acids as part of the "beta-oxidation" pathway, which shortens linear alkanoic acids in steps of C2-units, as shown in Figure 4.

Dioxygenases are enzymes catalysing reactions in which both oxygen atoms of O2 react with organic chemicals and appear to be unique to microorganisms such as bacteria. Examples of these reactions are shown for benzene in Figure 5. Similar reactions are involved in the degradation of more complex aromatic chemicals such as PAHs and halogenated aromatics.

The absence of oxygen in anaerobic environments (sediments and groundwater) does not preclude oxidation of organic chemicals. Other oxidants present (nitrate, sulphate, Fe(III) etc) may be present in sufficiently high concentrations to act as oxidants and terminal electron acceptors supporting microbial growth. In the absence of oxygen, activation relies on other reactions, the most important reactions seem to be carboxylation or addition of fumarate. Figure 6 shows an example of the degradation of naphthalene to CO2 in sediment microcosms under sulphate-reducing conditions.

Other important reactions in anaerobic ecosystems (sediments and groundwater plumes) are reductions. This affects functional groups, for example reduction of acids to aldehydes to alcohols, nitro groups to amino groups and, particularly important, substitution of halogens by hydrogen. The latter reactions can contribute to the conversion of highly chlorinated chemicals, that are resistant to oxidative biodegradation, to less chlorinated products which are more amenable to aerobic biodegradation. Many examples of these reductive dehalogenation reactions have been shown to occur in, for example, tetrachloroethene-contaminated groundwater (e.g. from dry-cleaning processes) and PCB-contaminated sediment. These reactions are exothermic under anaerobic conditions and some microorganisms are able to harvest this energy to support their growth. This can be considered to be a form of respiration based on dechlorination and is sometimes referred to as chlororespiration.
As is the case for abiotic degradation, hydrolyses are also important reactions in biodegradation pathways, particularly for chemicals that are derivatives of organic acids, such as carbamate, ester and organophosphate pesticides where hydrolyses are often the first step in their biodegradation. These reactions are similar to those described in the section on Chemical degradation.
References
Itrich, N.R., McDonough, K.M., van Ginkel, C.G., Bisinger, E.C., LePage, J.N., Schaefer, E.C., Menzies, J.Z., Casteel, K.D., Federle, T.W. (2015). Widespread microbial adaptation to L-glutamate-N,N,-diacetate (L-GLDA) following its market introduction in a consumer cleaning product. Environmental Science & Technology 49, 13314-13321.
Janssen, D. B., Dinkla, I. J. T., Poelarends, G. J., Terpstra, P. (2005). Bacterial degradation of xenobiotic compounds: evolution and distribution of novel enzyme activities, Environmental Microbiology 7, 1868-1882.
Kleemann, R., Meckenstock, R.U. (2017). Anaerobic naphthalene degradation by Gram-positive, iron-reducing bacteria. FEMS Microbial Ecology 78, 488-496.
Schwarzenbach, R.P., Gschwend, P.M., Imboden, D.M. (2017). Environmental Organic Chemistry, Third Edition, Wiley, ISBN 978-1-118-76723-8
Van Leeuwen, C., Vermeire, T.G. (2007). Risk Assessment of Chemicals: An Introduction (2nd ed.), Springer, ISBN 978-1-4020-6101-1
Zhou, Q., Chen, L. C., Wang, Z., Wang, J., Ni, S., Qiu, J., Liu, X., Zhang, X., Chen, X. (2017). Fast atrazine degradation by the mixed cultures enriched from activated sludge and analysis of their microbial community succession. Environmental Science & Pollution Research 24, 22152-22157.
Degradation by microorganisms plays an important role in the environmental fate of industrial organic chemicals. Explain briefly why the role of microorganisms is so important.
Biodegradation rates depend on amongst other factors on the structure of the chemicals. Mention three structural factors responsible for slow biodegradation.
DDT is one of the original "dirty dozen" Persistent Organic Pollutants (POPs). Explain what these POPs are and why they are labelled as persistent. What structural features are responsible for them being labelled as POPs?
Groundwater used to prepare drinking water is discovered to be contaminated with toluene and tetrachloroethene. Although nothing is known about the geochemical conditions in the groundwater aquifer, you are asked to investigate whether there is any evidence for biodegradation of these compounds occurring in the aquifer. Suggest which compounds could be analysed as evidence for biodegradation.
3.7.3. Degradation test methods
Authors: John Parsons
Reviewers: Steven Droge, Russell Davenport
Leaning objectives:
You should be able to:
- explain the strategy used in standardised biodegradability testing
- describe the most important aspects of standard biodegradability testing protocols
- interpret the results of standardised biodegradability tests
Keywords: Environmental fate, chemical degradation, photochemical degradation, biodegradation, mineralisation, degradation rate
Introduction
Many experimental approaches are possible to measure the environmental degradation of chemicals, ranging from highly controlled laboratory experiments to environmental monitoring studies. While each of these approaches has its advantages and disadvantages, a standardised and relatively straightforward set of protocols has clear advantages such as suitability for a wide range of laboratories, broad scientific and regulatory acceptance and comparability for different chemicals.
The system of OECD test guidelines (see links in the reference list of this chapter) is the most important set of standardised protocols although other test systems may be used in other regulatory contexts. As well as tests covering environmental fate processes, they also cover physical-chemical properties, bioaccumulation, toxicity etc. These guidelines have been developed in an international context and are adopted officially after extensive validation and testing in different laboratories. This ensures their wide acceptance and application in different regulatory contexts for chemical hazard and risk assessment.
Chemical degradation tests
The OECD Guidelines include only two tests specific for chemical degradation. This might seem surprising but it should not be forgotten that chemical degradation could also contribute to the removal observed in biodegradability tests. The OECD Guidelines for chemical degradation are OECD Test 111: Hydrolysis as a Function of pH (OECD 2004) and OECD Test 316: Phototransformation of Chemicals in Water - Direct Photolysis (OECD 2008). If desired, sterilised controls may also be used to determine the contribution of chemical degradation in biodegradability tests.
OECD Test 111 measures hydrolytic transformations of chemicals in aquatic systems at pH values normally found in the environment (pH 4 - 9). Sterile aqueous buffer solutions of different pH values (pH 4, 7 and 9) containing radio-labelled or unlabelled test substance (below saturation) are incubated in the dark at constant temperature and analysed after appropriate time intervals for the test substance and for hydrolysis products. The preliminary test is carried out for 5 days at 50°C and pH 4.0, 7.0 and 9.0, this is known as a first tier test. Further second tier tests study the hydrolysis of unstable substances and the identification of hydrolysis products and may extend for 30 days.
OECD Test 316 measures direct photolysis rate constants using xenon arc lamp capable of simulating natural sunlight in the 290 to 800 nm or natural sunlight, and extrapolated to natural water. If estimated losses are superior or equal to 20%, the transformation pathway and the identities, concentrations, and rate of formation and decline of major transformation products are identified.
Biodegradability tests
Biodegradation is in general considered to be the most important removal process for organic chemicals in the environment and it is therefore no surprise that biodegradability testing plays a key role in the assessing the environmental fate and subsequent exposure risks of chemicals. Biodegradation is an extensively researched area but data from standardised tests are favoured for regulatory purposes as they are assumed to yield reproducible and comparable data. Standardised tests have been developed internationally, most importantly under the auspices of the OECD and are part of the wider range of tests to measure physical-chemical, environmental and toxicological properties of chemicals. An overview of these biodegradability tests is given in Table 1.
The way that biodegradability testing is implemented can vary in detail depending on the regulatory context but in general it is based on a tiered approach with all chemicals being subjected to screening tests to identify chemicals that can be considered to be readily biodegradable and therefore removed rapidly from wastewater treatment plants (WWTPs) and the environment in general. These tests were originally developed for surfactants and often use activated sludge from WWTPs as a source of microorganisms since biodegradation during wastewater treatment is a major conduit of chemical emissions to the environment. The so-called ready biodegradability tests are designed to be stringent with low bacterial concentrations and the test chemical as the only potential source of carbon and energy at high concentrations. The assumption is that chemicals that show rapid biodegradation under these unfavourable conditions will always be degraded rapidly under environmental conditions. Biodegradation is determined as conversion to CO2 (mineralisation), either by directly measuring CO2 produced, or the consumption of oxygen, or removal of dissolved organic carbon, as this is the most desirable outcome of biodegradation. The results that have to be achieved for a chemical to be considered readily biodegradable vary slightly depending on the test, but as an example in the OECD 301D test (OECD 2014), the consumption of oxygen should reach 70% of that theoretically required for complete mineralisation within 28 days.
Table 1. The OECD biodegradability tests
|
OECD TEST GUIDELINE |
PARAMETER MEASURED |
REFERENCE |
|
Ready biodegradability tests |
||
|
301A: DOC Die-away test |
DOC |
OECD 1992a |
|
301B: CO2 evolution test |
CO2 |
OECD 1992a |
|
301C: Modified MITI(I) test |
O2 |
OECD 1992a |
|
301D: Closed bottle test |
O2 |
OECD 1992a |
|
301E: Modified OECD screening test |
DOC |
OECD 1992a |
|
301F: Manometric respirometry test |
O2 |
OECD 1992a |
|
306: Biodegradability in seawater |
DOC |
OECD 1992c |
|
310: Test No. 310: Ready Biodegradability - CO2 in sealed vessels (Headspace Test). |
CO2 |
OECD 2014 |
|
Inherent biodegradability tests |
||
|
302A: Modified Semi-continuous Activated Sludge (SCAS) test |
DOC |
OECD 1981b |
|
302B: Zahn-Wellens test |
DOC |
OECD 1992b |
|
302C: Modified MITI(II) test |
O2 |
OECD 2009 |
|
Simulation tests |
||
|
303A: Activated sludge units |
DOC |
OECD 2001 |
|
303B: Biofilms |
DOC |
OECD 2001 |
|
304A: Inherent biodegradability in soil |
14CO2 |
OECD 1981a |
|
307: Aerobic and anaerobic transformation in soil |
14CO2/CO2 |
OECD 2002a |
|
308: Aerobic and anaerobic transformation in aquatic sediment systems |
14CO2/CO2 |
OECD 2002b |
|
309: Aerobic mineralization in surface water |
14CO2/CO2 |
OECD 2004b |
|
311: Anaerobic biodegradability of organic compounds in digested sludge: by measurement of gas production |
CO2 and CH4 |
OECD 2006 |
|
314: Simulation tests to assess the biodegradability of chemicals discharged in wastewater |
Concentration of chemical, 14CO2/CO2 |
OECD 2008a |
These test systems are widely applied for regulatory purposes but they do have a number of issues. These include the fact that there are practical difficulties when applied to volatile or poorly soluble chemicals, but probably the most important is that for some chemicals the results can be highly variable. This is usually attributed to the source of the microorganisms used to inoculate the system. For many chemicals, there is a wide variability in how quickly they are degraded by activated sludge from different WWTPs. This is probably the result of different exposure concentrations and exposure periods to the chemicals, and may also be caused by dependence on the ability of small populations of degrading microorganisms, which may not always be included in the sludge samples used in the tests. These issues are not dealt with in any systematic way in biodegradability testing. It has been suggested that a preliminary period of exposure to the chemicals to be tested would allow sludge to adapt to the chemicals and may yield more reproducible test results. Further suggestions include using a higher, more environmentally relevant, concentration of activated sludge as the inoculum.
Failure to comply with the pass criteria in ready biodegradability tests does not necessarily mean that the chemical is persistent in the environment since it is possible that slow biodegradation may occur. These chemicals may therefore be tested further in higher tier tests, for what is referred to as inherent biodegradability in tests performed under more favourable conditions or in simulation tests representing specific compartments, to determine whether biodegradation may contribute significantly to their removal. These tests are also standardised (see Table 1). Simulation tests are designed to represent environmental conditions in specific compartments, such as redox potential, pH, temperature, microbial community, concentration of test substance and occurrence and concentration of other substrates.
The criteria used in classifying the biodegradability of chemicals depend on the regulatory context. Biodegradability tests can be used for different purposes: in the EU this includes 3 distinct purposes; classification and labelling, hazard/persistent assessment, and environmental risk assessment Recently regulatory emphasis has shifted to identifying hazardous chemicals, and therefore those chemicals that are less biodegradable and likely to persist in the environment. Examples for the classification as PBT (persistent, bioaccumulative and toxic) or vPvB (very persistent and very bioaccumulative) chemicals are shown in Table 2. As well as the results of standardised tests, other data such as the results of environmental monitoring data or studies on the microbiology of biodegradation can also be taken into account in evaluations of environmental degradation in a so-called weight of evidence approach.
Table 2. Criteria used to classify chemicals as PBT or vPvB (van Leeuwen & Vermeire 2007)
|
Property |
PBT criteria |
vPvB criteria |
|
Persistence |
T1/2 >60 days in marine water, or T1/2 >40 days in fresh/estuarine water, or T1/2 >180 days in marine sediment, or T1/2 >120 days in fresh/estuarine sediment, or T1/2 >120 days in soil. |
T1/2 >60 days in marine, fresh or estuarine water, or T1/2 >180 days in marine, fresh or estuarine sediment, or T1/2 >180 days in soil |
|
Bioaccumulation |
BCF > 2000 L/kg |
BCF > 5000 L/kg |
|
Toxicity |
- NOEC < 0.01 mg/L for marine or freshwater organisms, or - substance is classified as carcinogenic, mutagenic, or toxic for reproduction, or - there is other evidence of chronic toxicity, according to Directive 67/548/EEC |
The results of biodegradability tests are sometimes also used to derive input data for environmental fate models (see section on Multicompartment modeling). It is however not always straightforward to transfer data measured in what is sometimes a multi-compartment test system into degradation rates in individual compartments as other processes (e.g. partitioning) need to be taken into account.
References
OECD, 1981a. OECD Guidelines for the Testing of Chemicals. Test No. 304A: Inherent Biodegradability in Soil.
OECD, 1981b. OECD Guidelines for the Testing of Chemicals. Test No. 302A: Inherent Biodegradability: Modified SCAS Test.
OECD, 1992a. OECD Guidelines for the Testing of Chemicals. Test No. 301: Ready Biodegradability.
OECD, 1992b. OECD Guidelines for the Testing of Chemicals. Test No. 302B: Inherent Biodegradability: Zahn-Wellens/ EVPA Test.
OECD, 1992c. OECD Guidelines for the Testing of Chemicals. Test No. 306: Biodegradability in Seawater.
OECD, 2001. OECD Guidelines for the Testing of Chemicals. Test No. 303: Simulation Test - Aerobic Sewage Treatment - A: Activated Sludge Units; B: Biofilms.
OECD, 2002a. OECD Guidelines for the Testing of Chemicals. Test No. 307: Aerobic and Anaerobic Transformation in Soil.
OECD, 2002b. OECD Guidelines for the Testing of Chemicals. Test No. 308: Aerobic and Anaerobic Transformation in Aquatic Sediment Systems.
OECD, 2004a. OECD Guidelines for the Testing of Chemicals. Test No. 111: Hydrolysis as a Function of pH.
OECD, 2004b. OECD Guidelines for the Testing of Chemicals. Test No. 309: Aerobic Mineralisation in Surface Water - Simulation Biodegradation Test
OECD, 2006. OECD Guidelines for the Testing of Chemicals. Test No. 311: Anaerobic Biodegradability of Organic Compounds in Digested Sludge: by Measurement of Gas Production.
OECD, 2008a. OECD Guidelines for the Testing of Chemicals. Test No. 314: Simulation Tests to Assess the Biodegradability of Chemicals Discharged in Wastewater
OECD, 2008b. OECD Guidelines for the Testing of Chemicals. Test No. 316: Phototransformation of Chemicals in Water - Direct Photolysis.
OECD, 2014. OECD Guidelines for the Testing of Chemicals. Test No. 310: Ready Biodegradability - CO2 in sealed vessels (Headspace Test).
Van Leeuwen, C.J., Vermeire, T.G. (2007). Risk Assessment of Chemicals: An Introduction (2nd ed.), Springer, ISBN 978-1-4020-6101-1
The OECD has published extensive protocols that can be used to evaluate the degradability of chemicals in the environment. Which forms of abiotic (chemical) degradation do these include?
Biodegradation tests used to evaluate the environmental effects of chemicals often measure the mineralisation of the chemicals, for example by measuring the amount of carbon dioxide produced. Explain why this is preferred to measuring the removal of the original chemical (primary biodegradation).
Assessment of the biodegradability of chemicals often follows a tiered system in which they are first screened for their ready biodegradability before undergoing more extensive testing to assess their inherent biodegradability or biodegradation in specific environmental compartments using simulation tests. Explain why this approach is applied.


