Vitamin A: β-Carotene
- Page ID
- 504
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
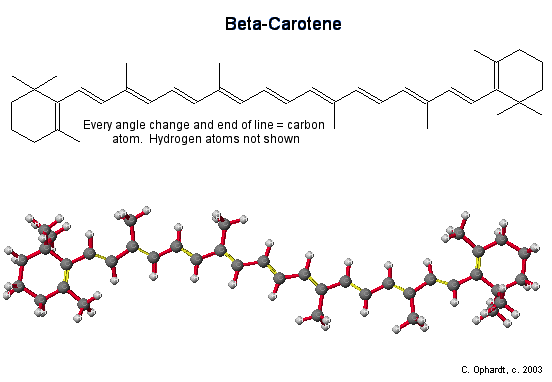
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)β-carotene is the molecule that gives carrots, sweet potatoes, squash, and other yellow or orange vegetables their orange color. It is part of a family of chemicals called the carotenoids, which are found in many fruit and vegetables, as well as some animal products such as egg yolks. Carotenoids were first isolated in the early 19th century, and have been synthesized for use as food colorings since the 1950s. Biologically, β-carotene is most important as the precursor of vitamin A in the human diet. It also has anti-oxidant properties and may help in preventing cancer and other diseases.
Introduction
The long chain of alternating double bonds (conjugated) is responsible for the orange color of beta-carotene. The conjugated chain in carotenoids means that they absorb in the visible region - green/blue part of the spectrum. So β-carotene appears orange, because the red/yellow colors are reflected back to us.
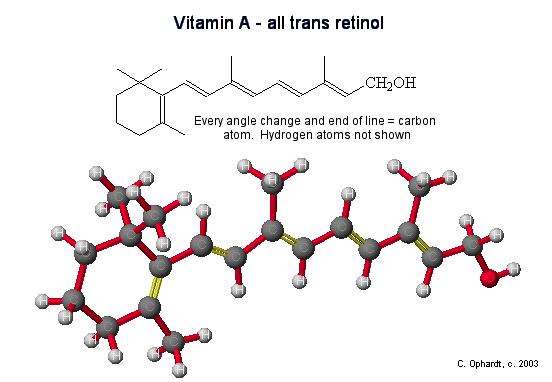
Vitamin A
Vitamin A has several functions in the body. The most well known is its role in vision - hence carrots "make you able to see in the dark". The retinol is oxidized to its aldehyde, retinal, which complexes with a molecule in the eye called opsin. When a photon of light hits the complex, the retinal changes from the 11-cis form to the all-trans form, initiating a chain of events which results in the transmission of an impulse up the optic nerve. A more detailed explanation is in Photochemical Events.
Other roles of vitamin A are much less well understood. It is known to be involved in the synthesis of certain glycoproteins, and that deficiency leads to abnormal bone development, disorders of the reproductive system, xerophthalmia (a drying condition of the cornea of the eye) and ultimately death.
Vitamin A is required for healthy skin and mucus membranes, and for night vision. Its absence from diet leads to a loss in weight and failure of growth in young animals, to the eye diseases; xerophthalmia, and night blindness, and to a general susceptibility to infections. It is thought to help prevent the development of cancer. Good sources of carotene, such as green vegetables are good potential sources of vitamin A. Vitamin A is also synthetically manufactured by extraction from fish-liver oil and by synthesis from beta-ionone.
Vitamin A is structurally related to β-carotene. β-Carotene is converted into vitamin A in the liver. Two molecules of vitamin A are formed from on molecule of beta carotene.

Oxidation: If you compare the two molecules, it is clear that vitamin A (retinol) is very closely related to half of the beta-carotene molecule. One way in which beta-carotene can be converted to vitamin A is to break it apart at the center and is thought to be most important biologically. The breakdown of beta-carotene occurs in the walls of the small intestine (intestinal mucosa) and is catalyzed by the enzyme β-carotene dioxygenase to form retinal.
Reduction Reaction: The retinal reduced to retinol by retinaldehyde reductase in the intestines. This is the reduction of an aldehyde by the addition of hydrogen atoms to make the alcohol, retinol.
Esterification Reaction: The absorption of retinol from the alimentary tract is favored by the simultaneous absorption of fat or oil, especially if these are unsaturated. Retinol is esterified to palmitic acid and delivered to the blood via chylomicrons. Finally the retinol formed is stored in the liver as retinyl esters. This is why cod liver oil used to be taken as a vitamin A supplement. It is also why you should never eat polar bear liver if you run out of food in the Arctic; vitamin A is toxic in excess and a modest portion of polar bear liver contains more than two years supply! Beta-carotene, on the other hand, is a safe source of vitamin A. The efficiency of conversion of beta-carotene to retinol depends on the level in the diet. If you eat more beta-carotene, less is converted, and the rest is stored in fat reserves in the body. So too much beta-carotene can make you turn yellow, but will not kill you with hypervitaminosis.
Contributors
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook

