Adrenergic Drugs
- Page ID
- 89062
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The compounds ordinarily classified as central stimulants are drugs that increase behavioral activity, thought processes, and alertness or elevate the mood of an individual. These drugs differ widely in their molecular structures and in their mechanism of action. Thus, describing a drug as a stimulant does not adequately describe its medicinal chemistry. The convulsions induced by a stimulant such as strychnine, for example, are very different from the behavioral stimulation and psychomotor agitation induced by a stimulant such as amphetamine.
A. Adrenergic Concepts.
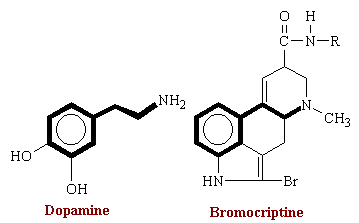
The three main catacholamines (chatecol is ortho-dihydroxybenzene) are epinephrine EP, norepinephrine NE, and dopamine DA. A host of physiological and metabolic responses follows stimulation of sympathetic nerves in mammals is usually mediated by the neurotransmitter norepinephrine. As part of the response to stress, the adrenal medulla is also stimulated, resulting in elevation of the concentrations of EP and NE in the circulation. The actions of these two catecholamine are very similar at some sites but differ in significantly at others. For example, both compounds stimulate the myocardium; however, EP dilates blood vessels to skeletal muscle, whereas NE has a minimal constricting effect on them. DA is found predominantly in the basal ganglia of the CNS and is found in very low levels in peripheral tissues.
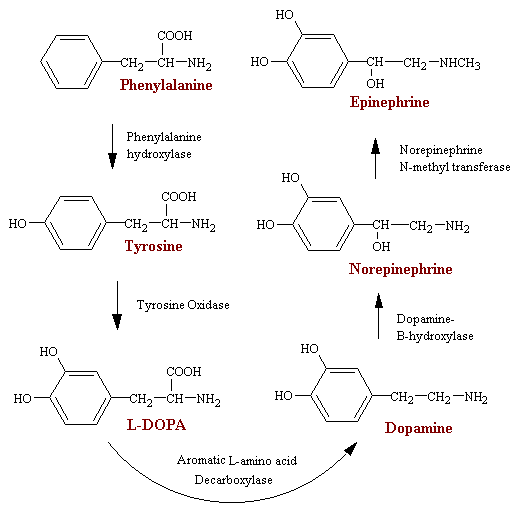
Synthesis of Catecholamines
The synthesis of the neurotransmitters DA and NE and EP and the hormones NE and EP takes place by a pathway that involves 5 enzymes (see figure below). Tyrosine is generally considered the starting point, although phenylalanine hydroxylase can hydroxylate phenylalanine to tyrosine in the event that there is a tyrosine deficiency. Tyrosine hydroxylase (structure) is the rate-limiting enzyme in this pathway. Its addition of the 3-OH yielding L-3, 4-dihydroxyphenylalanine (L-DOPA) requires O2, tetrahydropteridine, and Fe2+ as cofactors. One of the oxygen atoms in O2 is incorporated into an organic substrate and the other is reduced to water. Because this is the rate-limiting step, inhibition of this enzyme is the most likely way to reduce NE, DA, or EP levels significantly. Particularly are the a-methyltyrosine analogs, especially those containing an iodine atom in the benzene ring. The drug a -methyltyrosine is useful in the management of malignant hypertension and in pheochromocytoma. The latter is a chromaffin cell tumor that produces and spills copious amounts of NE and EP into the circulation.
DOPA is then converted to dopamine by the enzyme DOPA decarboxylase. The cofactor for this enzyme is pyridoxal (the aldahyde form of pyridoxine, vitamin B6). The copper-containing enzyme dopamine-beta-monooxygenase then converts dopamine to NE and in the end norepinephrine N-methyltransferase converts NE to EP.
Genetic defaults in, or complete absence of, the first of these 5 enzymes (Phenylalanine Hydroxylase) leads to a disease called phenylketoneuria PKU, which will lead to severe mental disorder if not treated at an early stage after birth.
Adrenergic Receptors
Research experiments using different drugs that mimic the action of norepinephrine on sympathetic effector organs have shown that there are two major types of adrenergic receptors, alpha receptors and beta receptors. The beta receptors in turn are divided into beta1 and beta 2 receptors because certain drugs affect only some beta receptors. Also, there is a less distinct division of alpha receptors into alpha1 and alpha 2 receptors.
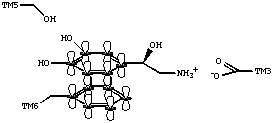
Just as in the muscarinic receptor, and most other G protein-coupled receptors that bind biogenic amines, the adrenergic receptors possess an aspartate residue in the third transmembrane domain. The aspartate residue appears to interact with the amine residue of norepinephrine and other adrenergic ligands. Conserved serine residues in TM5 may play a role in the binding of adrenergic ligands through hydrogen bond interactions. In addition, aromatic amino acid residues, such as a phenylalanine in TM6, may contribute to the binding of ligands through pi - pi interactions.
Norepinephrine and epinephrine, both of which are secreted into the blood by the adrenal medulla, have somewhat different effects in exciting the alpha and beta receptors. Norepinephrine excites mainly alpha receptors but excites the beta receptors to a less extent as well. On the other hand, epinephrine excites both types of receptors approximately equally. Therefore, the relative effects of norepinephrine and epinephrine on different effector organs are determined by the types of receptors in the organs. If they are all beta receptors, epinephrine will be the more effective excitant. It should be emphasised that not all tissues have both of these receptors. Usually they are associated with only one type of receptor or the other.
| The relative potencies of the various receptors are: | |
|---|---|
| a -adrenergic receptors: | EP > NE |
| b -adrenergic receptors: | EP = NE |
| b 1-adrenergic receptors (heart): | EP = NE |
| b 2-adrenergic receptors (most other tissues): | EP >> NE |
EP dilates blood vessels (relaxes smooth muscle) in skeletal muscle and liver vascular beds; NE constricts the same vascular beds. EP decreases resistance in the hepatic and skeletal vascular smooth muscle beds; NE increases resistance. In contrast to their opposite effects on vascular smooth muscle of the liver and skeletal muscle, both EP and NE cause vasoconstriction (contraction of smooth muscle) in blood vessels supplying the skin and mucosa. EP decreases diastolic blood pressure; NE increases diastolic blood pressure. EP relaxes bronchial smooth muscle; NE has little effect. Both EP and NE stimulate an increased rate of beating when applied directly to a heart muscle removed from the body and isolated from nervous input. In contrast, NE given intravenously causes a profound reflex bradycardia due to a baroreceptor/vagal response (and increased release of acetylcholine onto the heart) in response to the vasopressor effect of NE.
| a -receptor | b -receptor |
|---|---|
| vasocontriction | vasodilation (b 2) |
| iris dilation | cardioacceleration (b 1) |
| intestinal relaxation | intestinal relaxation (b 2) |
| intestinal sphincter contraction | uterus relaxation(b 2) |
| bladder sphincter contraction | bronchiodilation (b 2) |
The binding of the adrenergic receptor causes a series of reactions that eventually results in a characteristic response.
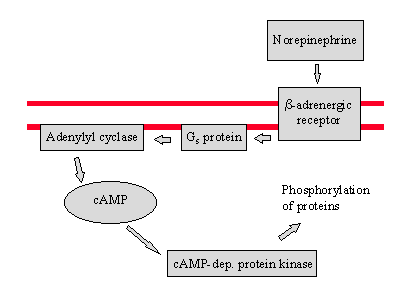
Two of the proteins that are phosphorylated in this process breakdown glycogen and stop glycogen synthesis.
Catabolism of Catecolamines
There are three main ways in which catacolamines are removed from a receptor - recycling back into the presynaptic neuron by an active transport reuptake mechanism, degredation to inactive compounds through the sequential actions of catecholamine-O-methyltransferase (COMT) and monoamine oxidase (MAO), and simple diffusion (see figure below).
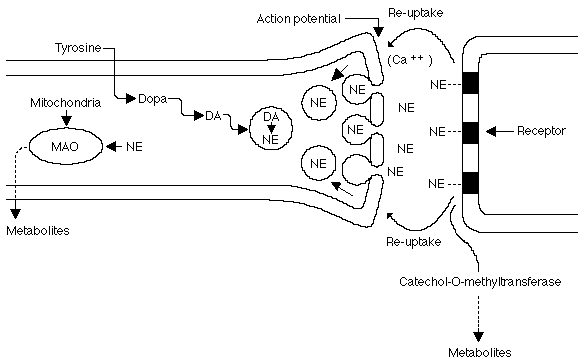
Schematic representation of an adrenergic junction. Copyright © 1996-1997 Merck & Co., Inc., Whitehouse Station, NJ, USA. All rights reserved.
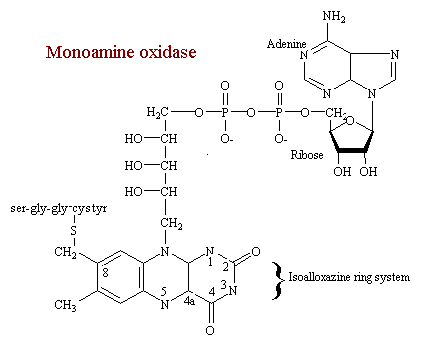
Monoamine Oxidase
MAO catalyzes the oxidative deamination of catecholamines, serotonin, and other monoamines. It is one of several oxydase-type enzymes who's coenzyme is the flavin-adenine-dinucleatide (FAD) covalently bound as a prosthetic group. The isoallozazine ring system is viewed as the catalytically functional component of the enzyme. In this view N-5 and C-4a is where the redox reaction takes place. Although the whole region undoubtedly participates.
Norepinephrine (NE) is the neurotransmitter of most postganglionic sympathetic fibers and many central neurons (eg, locus ceruleus, hypothalamus). Upon release, NE interacts with adrenergic receptors. This action is terminated largely by the re-uptake of NE back into the prejunctional neurons. Tyrosine hydroxylase and MAO regulate intraneuronal NE levels. Metabolism of NE occurs via MAO and catechol-O-methyltransferase to inactive metabolites (eg, normetanephrine, 3-methoxy-4-hydroxyphenylethylene glycol, 3-methoxy-4-hydroxymandelic acid).
B. Stimulants
Epinephrine
Epinephrine is a potent stimulator of both a and b -adrenergic receptors, and its effects on target organs are thus complex. Most of the effects which occur after injection are listed in the table on a and b -receptors shown above. Particularly prominent are the actions on the heart and the vascular and other smooth muscle.
Epinephrine is one of the most potent vasopressor drugs known. Given intravenously it evokes a characteristic effect on blood pressure, which rises rapidly to a peak that is proportional to the dose. The increase is systolic pressure is greater than diastolic pressure, so that the pulse pressure increases. As the response wanes, the mean pressure falls below normal before returning to normal. The mechanism of the rise in blood pressure due to epinephrine is three fold; a direct myocardial stimulation that increases the strength of ventricular contraction; and increased heart rate; and most important, vasoconstriction in many vascular beds, especially the in the vessels of the skin, mucosa, and kidney, and constriction in the veins. Due to this increased blood pressure and to powerful b 2-receptor vasodilator action that is partially counterbalanced by vasoconstrictor action on the a receptors that are also present, blood flow to the skeletal muscles and central nervous system is increased.
The effects of epinephrine on the smooth muscles of different organs and systems depend upon the type of adrenergic receptor in the muscle. It has powerful bronchiodilatior action, most evident when bronchial muscle is contracted as in bronchial asthma. In such situations, epinephrine has a striking therapeutic effect as a physiological antagonist to the constrictor influences since it is not limited to specific competitive antagonism such as occurs with antihistaminic drugs against histamine-induced bronchiospasm.
Epinephrine has a wide variety of clinical uses in medicine and surgery. In general, these are based on the actions of the dug on blood vessels, heart, and bronchial muscle. The most common uses of epinephrine are to relieve respiratory distress due to bronchiospasm and to provide rapid relief of hypersensitivity reactions to drugs and other allergens. Its cardiac effects may be of use in restoring cardiac rhythm in patients with cardiac arrest. It is also used as a topical hemostatic on bleeding surfaces.
Norepinephrine
Norepinephrine is the chemical mediator liberated by mammalian postgangionic adrenergic nerves. It differs from epinephrine only by lacking the methyl substitution in the amino group. Norepinephrine constitutes 10 to 20% of the catecholamine content of human adrenal medulla. Norepinephrine is a potent agonist at a receptors and has little action on b 2 receptors; however, it is somewhat less potent than epinephrine on the a receptors of most organs. Most of the effects which occur after injection are listed in the table on a and b -receptors shown above Norepinephrine has only limited therapeutic value.
Amphetamine and Methamphetamine
Amphetamine, racemic b-phenylisopropylamine, has powerful CNS stimulant actions in addition to the peripheral a and b actions common to indirectly acting sympathomimetic drugs. Unlike epinephrine, it is effective after oral administration and its effects last for several hours. Although amphetamine and methamphetamine are almost structurally identical to norepinephrine and epinephrine, these drugs have an indirect sympathomimetic action rather than directly exciting adrenergic effector receptors. Their effect is to cause release of norepinephrine from its storage vesicles in the sympathetic nerve endings The release of norepinephrine in turn causes the sympathetic effects.
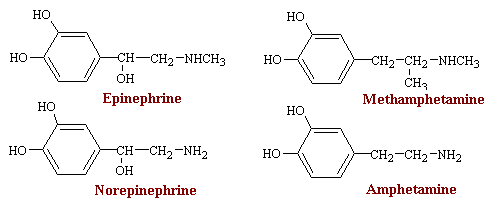
Ephedrine
Ephedrine occurs naturally in various plants. It was used in China for at least 2000 years before being introduced into Western medicine in 1924. Its central actions are less pronounced than those of the amphetamines. Ephedrine stimulates both a and b receptors and has clinical uses related to both these types of action. The drug owes part of its peripheral action to the release of norepinephrine, but it also has direct effects of receptors.
Since ephedrine contains two chiral carbon atoms, four compounds are possible. Clinically, D-ephedrine is used to a large extent as an anti-asthmatic and, formerly, as a presser amine to restore low blood pressure as a result of trauma. L-pseudo-ephedrine is used primarily as a nasal decongestant.
Ephedrine differs from epinephrine mainly in its efficacy after oral administration, its much longer duration of action, its more pronounced central actions, and its much lower potency. Cardiovascular effects of ephedrine are in many ways similar to those of epinephrine, but they persist about ten times as long. The drug elevates the systolic and diastolic pressure in man, and pulse pressure increases. Bronchial muscle relaxation is less prominent but more sustained with ephedrine than with epinephrine.
The main clinical uses of ephedrine are in bronchiospasm, as a nasal decongestant, and certain allergic disorders, The drug is also used, although perhaps unwisely, as a weight loss agent.
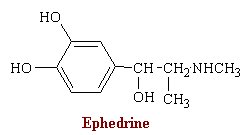
MAOIs
The monoamine oxidase inhibitors (MAOIs) comprise a chemically heterogeneous group of drugs that have in common the ability to block oxidative deamination of naturally occurring monoamines. These drugs have numerous other effects, many of which are still poorly understood. For example, they lower blood pressure and were at one time used to treat hypertension. Their use in psychiatry has also become very limited as the tricyclic antidepressants have come to dominate the treatment of depression and allied conditions. Thus, MAOIs are used most often when tricyclic antidepressants give unsatisfactory results. In addition, whereas severe depression may not be the primary indication for these agents, certain neurotic illnesses with depressive features, and also with anxiety and phobias, may respond especially favorably.
Two main problems are associated with the MAOIs. The first is that an amino acid called "tyramine" may cause a hypertensive reaction in some people taking MAOIs. Therefore, foods containing tyramine must be avoided. Alcohol and caffeine must also be eliminated from the diet. Certain medications may react dangerously when combined with MAOIs. Therefore, it is crucial to tell the prescribing doctor about medications (including over-the-counter) you are taking. The second problem associated with MAOIs is the possibility of side effects. MAOIs not only inhibit MAO but other enzymes as well, and they interfere with the hepatic metabolism of many drugs. The dietary restrictions and side effects deter many people from staying on MAOIs.
Phenelzine is the hydrazine analog of phenylethylamine, a substrate of MAO. This and several other MAOIs, such as isocarboxazide, are structurally related to amphetamine and were synthesized in an attempt to enhance central stimulant properties.
Cocaine
Cocaine blocks the reuptake of dopamine by presynaptic neurons. More about this can be found under the topic Illegal Drugs.
Dopamine
Dopamine is the immediate metabolic precursor of NE and EP; it is a central neurotransmitter and possesses important intrinsic pharmacological properties. DA is a substrate for both MAO and COMT and thus is ineffective when administered orally.
Parkinson's Disease. Parkinson's disease can be characterized as having a DA deficiency in the brain. The pathology can be traced to certain large neurons in the substantia nigra in the basal ganglia, whose degeneration is directly related to DA deficiency. One of the principle roles of the basal ganglia is to control complex patterns of motor activity. When there is damage to the basal ganglia one's writing becomes crude.
Logic would dictate that increasing brain levels of DA should ameliorate symptoms of Parkinson's disease. Direct parental DA administration is useless since the compound does not penetrate the Blood-brain barrier. It is shown that oral dosing with L-DOPA can successfully act as a pro-drug to the extent it enters the brain and is then decarboxylated to DA there. The clinical results in terms of decreased tremors and rigidity are dramatic. However, there are complications which produce intense side effects including nausea and vomiting, that are presumably due to chemoreceptor trigger zone stimulation by large amounts of DA produced peripherally. The reason for this situation is the relatively high peripheral levels of decarboxylase enzyme compared with brain concentrations. Thus 95% of a given oral dose was converted to DA before reaching the brain to be decarboxylated there. This can be prevented using L-DOPA in combination with a drug called carbidopa (more).
Amantadine, introduced as an antiviral agent for the influenza was unexpectedly found to cause symptomatic improvement of patients with parkinsonism. Amantadine is a basic amine like dopamine, but the lipophilic nature of the cage structure enhances its ability to cross the blood brain barrier. This drug acts by releasing dopamine from intact dopaminergic terminals that remain in the nigrostraeatum of patients with Parkinson's disease. Because of this facilitated release of dopamine it appears that the therapeutic efficacy of amantadine is enhanced by the concurrent administration of levodopa. Amantadine has also been shown to delay the re-uptake of dopamine by neural cells, and it may have anticholinergic effects as well. A three dimensional view of amantadine may provide a better understanding of the structure.
The above therapies are based on the manipulation of endogenous stores of dopamine. Dopamine agonists can stimulate the receptor directly and are of therapeutic value. Some of the drugs acting as dopaminergic agonists include the ergot alkaloid derivative bromocriptine. Bromocriptine is used particularly when L-DOPA therapy fails during the advanced stages of the disease. Bromocriptine is a derivative of lysergic acid (a precursor for LSD). Its structure is shown below. The addition of the bromine atom renders this alkaloid a potent dopamine agonist and virtually all of its actions result from stimulation of dopamine receptors.
Schizophrenia. Schizophrenia results from excessive excitement of a group of neurons that secrete dopamine in the behavioral centers of the brain, including in the frontal lobes. Therefore drugs used to treat this disorder decrease the level of dopamine excreted from these neurons or antagonize dopamine. We will discuss these drugs in detail later under the topic Psychoactive Drugs.
Strychnine
Strychnine does not directly affect adrenergic mechanisms, and technically should not be listed in this category. However, its stimulating affects are a result of adrenergic mechanisms. In addition, strychnine has no demonstrated therapeutic value, despite a long history of unwarranted popularity. However, the mechanism of action of strychnine is thoroughly understood, and it is a valuable pharmacological tool for studies of inhibition in the CNS. Poisoning with strychnine results in a predictable sequence of dramatic symptoms that may be lethal unless interrupted by established therapeutic measures.
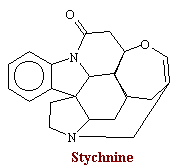
Strychnine is the principle alkaloid present in nux vomica, the seeds of a tree native to India, Stychnos nux-vomica. The structural formula for strychnine is:
Strychnine produces excitation of all portions of the CNS. This effect, however, does not result from direct synaptic excitation. Strychnine increases the level of neuronal excitability by selectively blocking inhibition. Nerve impulses are normally confined to appropriate pathways by inhibitory influences. When inhibition is blocked by strychnine, ongoing neuronal activity is enhanced and sensory stimuli produce exaggerated reflex effects.
Strychnine is a powerful convulsant, and the convulsion has a characteristic motor pattern. Inasmuch as strychnine reduces inhibition, including the reciprocal inhibition existing between antagonistic muscles, the pattern of convulsion is determined by the most powerful muscles acting at a given joint. In most laboratory animals, this convulsion is characterized by tonic extension of the body and of all limbs.
The convulsant action of strychnine is due to interference with post synaptic inhibition that is mediated by glycine. Glycine is an important inhibitory transmitter to motorneurons and interneurons in the spinal cord, and strychnine acts as a selective, competitive antagonist to block the inhibitory effects of glycine at all glycine receptors. Competitive receptor-binding studies indicate that both strychnine and glycine interact with the same receptor complex, although possibly at different sites.
The first symptoms of strychnine poisoning that is noticed is stiffness of the face and neck muscles. Heightened reflex excitability soon becomes evident. Any sensory stimulus may produce a violent motor response. In the early stages this is a coordinated extensor thrust, and in the later stages it may be a full tetanic convulsion. All voluntary muscles, including those of the face, are soon in full contraction. Respiration ceases due to the contraction of the diaphragm and the thoracic and abdominal muscles.

