1.9: Cheese Production
- Page ID
- 306246
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Cheese Production (University of Guelph, Cheese Production)
Cheese making is essentially a dehydration process in which milk casein, fat and minerals are concentrated 6 to 12-fold, depending on the variety. The basic steps common to most varieties are acidification, coagulation, dehydration, and salting.
Cheese Production Process:
Chemical Components of Milk
Milk is primarily composed of water with four biological macromolecules; carbohydrate (lactose), fats, casein phosphoproteins, and whey protein.
Casein
Caseins are phosphoproteins. These proteins are mostly random coils will little secondary or tertiary structure. They are highly heat stable.
- Define primary, secondary and tertiary structure in proteins.
- Circle the phosphorylated serine side chains in a typical repeating unit in \(\beta\)-casein.
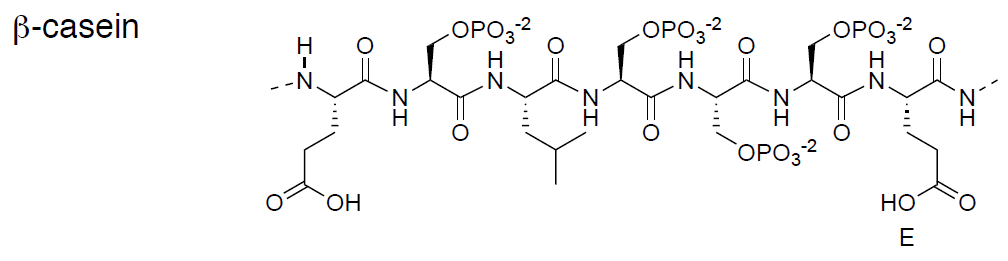
- Caseins have a relatively high [ negative / positive ] charge from phosphates.
Casein exists in the milk as micelles that consist of hundreds of casein molecules coordinated with Ca+2 ions.
- Draw a cartoon of several casein globular proteins forming a micelle with a hydrophobic center and a hydrophilic outer surface. Add the calcium ions.
Casein Aggregation (curd formation)
Although the casein micelle is fairly stable, here are two major ways in which aggregation can be induced. Aggregation is a key step of cheese production.
- Enzymatic - chymosin (rennet) or other enzymes (important for cheddars and gouda)
During the primary stage, rennet cleaves the Phe(105)-Met(106) linkage of kappa-casein forming a soluble protein which diffuses away from the micelle and para-kappa-casein.
During the secondary stage, the micelles aggregate. This is due to the loss of steric repulsion of the kappa-casein. Calcium assists coagulation by acting as a bridge between micelles.
During the tertiary stage of coagulation, a gel forms, the milk curd firms, and the liquid separates.
- Draw a cartoon of several micelles clumping together. Show the calcium ions acting as bridges.
2. Acid. Acidification causes the casein micelles to destabilize or aggregate. Acid coagulated fresh cheeses may include Cottage cheese, Quark, and Cream cheese.
- Considering the number of phosphate groups present, suggest what will happen to the phosphates as the pH drops below 4.6.
- Draw the acid-base reaction that occurs.
- What happens to the Ca+2 ions?
Acid coagulation can be achieved naturally with the starter culture of lactobacillus.
- These bacteria convert lactose to _________________.
Acid curd is more fragile than rennet curd due to the loss of calcium.
- Explain why the loss of calcium makes a more fragile curd.
Whey
Whey proteins include \(\beta\) -lactoglobulin, alpha-lactalbumin, bovine serum albumin (BSA), and immunoglobulins (Ig). These proteins have well defined tertiary and quaternary structures. They are soluble in water at lower pH but do not coagulate after proteolysis or acid treatment. When the casein is coagulated with enzymes or acid treatment, there is usually a straining step whereby the water is separated from the curd.
- Does the whey stay with the curd or the water?
There is a third process for casein coagulation, heat-acid.
In this process, heat causes denaturation of the whey proteins which can interact with the caseins. With the addition of acid, the caseins precipitate with the whey proteins.
- Draw a cartoon of this process.
In heat-acid coagulation, 90% of protein can be recovered. Examples of cheeses made by this method include Paneer, Ricotta and Queso Blanco.
Metabolism of Lactose in Homofermentative Lactobacilli
Overview
When lactobacilli are added to milk, the bacterium uses enzymes to produce energy (ATP) from lactose.
- The byproduct of ATP production is __________.
The lactic acid curdles the milk that then separates to form curds, which are used to produce cheese and whey.
We previously covered the pathway for bacteria to convert glucose to lactic acid.
- Recap this pathway and the reason that these bacteria use this process rather than TCA cycle and oxidative phosphorylation.
However, we haven’t talked about how this bacterium can convert lactose to glucose.
- Lactose is a [ monosaccharide / disaccharide / polysaccharide ]. Circle one.
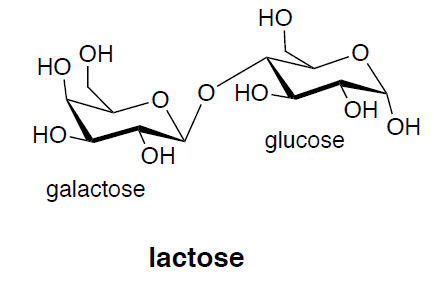
Lactose is hydrolyzed to glucose and \(\beta\)-galactose.
- Draw the two monosaccharides.
Galactose Metabolism
Glucose can be converted to lactic acid as discussed before. Galactose is converted into glucose 6-phosphate in four steps in the Leloir pathway.
Leloir Pathway Stepwise
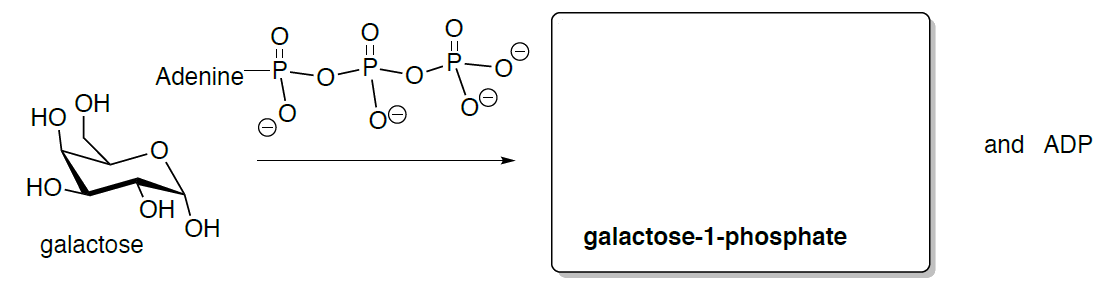
1. The first reaction is the phosphorylation of galactose to galactose 1-phosphate.
- Draw the reaction and predict the product for this reaction.
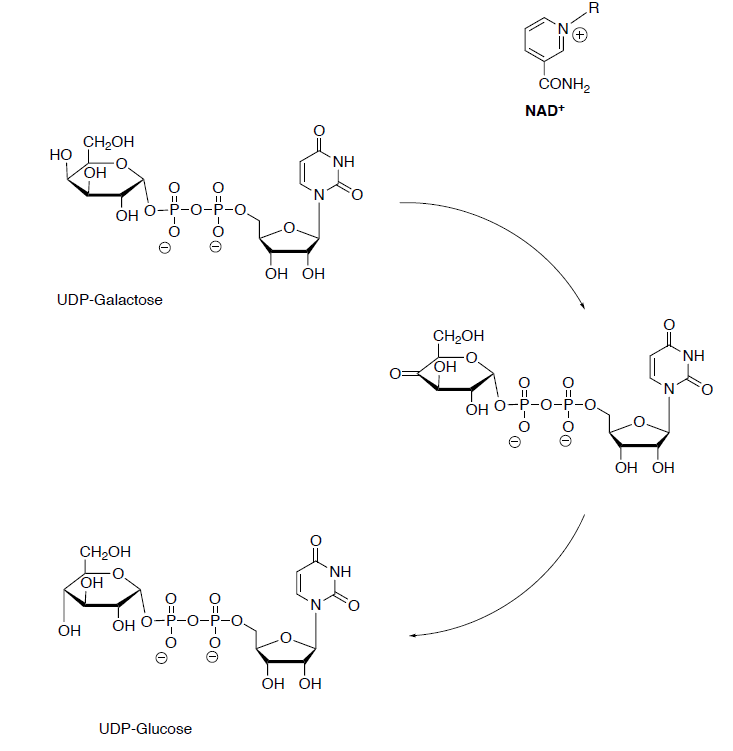
2. Galactose 1-phosphate reacts with uridine diphosphate glucose (UDP-glucose) to form UDP-galactose and glucose 1-phosphate are formed.
- Draw the reaction and predict the products for this reaction.
3. The galactose moiety of UDP-galactose is then epimerized to glucose-1-phosphate. The configuration of the hydroxyl group at carbon 4 is inverted by UDP-galactose 4-epimerase.
This enzyme utilizes NAD+ in the first step. And then regenerates the NAD+ in the second step.
- Draw the arrows for this reaction. Add in the NAD+.
4. Glucose 1-phosphate, formed from galactose, is isomerized to glucose 6-phosphate by phosphoglucomutase.
In this pathway, UDP-glucose and UDP-galactose fulfill catalytic roles but are not subject to any net turnover. It might therefore be said that they form a tiny metabolic cycle between the two of them.
- Explain this observation about the Leloir Pathway.
Maturation of Cheese
Preparation and Ripening
After the whey is removed the curds, there is a wide variety of curd handling dependent upon the type of cheese being prepared. Some cheese varieties, such as Colby or Gouda require a curd washing to increases the moisture content and reduce the acidity. Salt is added to some cheeses through different methods: Gouda is soaked in brine, while Feta has surface salt added.
The curd is then ripened until the desired flavors and textures are produced. This ripening process includes further fermentation by bacteria, added yeasts or molds, and enzymatic reactions from added lipases or rennet. These processes develop distinctive characteristics for each cheese.
The table below shows a sample of flavor molecules derived from the breakdown of milk
components.
| Casein Protein | Milk Fats | Lactose |
|---|---|---|
| Ethanoic Acid | Carboxylic Acids | Diacetyl |
| Aldehydes | Lactones | Acetaldehyde |
| Amines | Esters | Acetic Acid |
| Ketones |
More examples: Simon Cotton, Education in Chemistry, Royal Society of Chemistry, Really Cheesy Chemistry
Maturation of Cheese
Lipolysis
Lipolysis is a critical step is the lipolysis of triglycerides to esters and acids which yield many flavorful molecules.
- Draw the products of this lipolysis reaction.
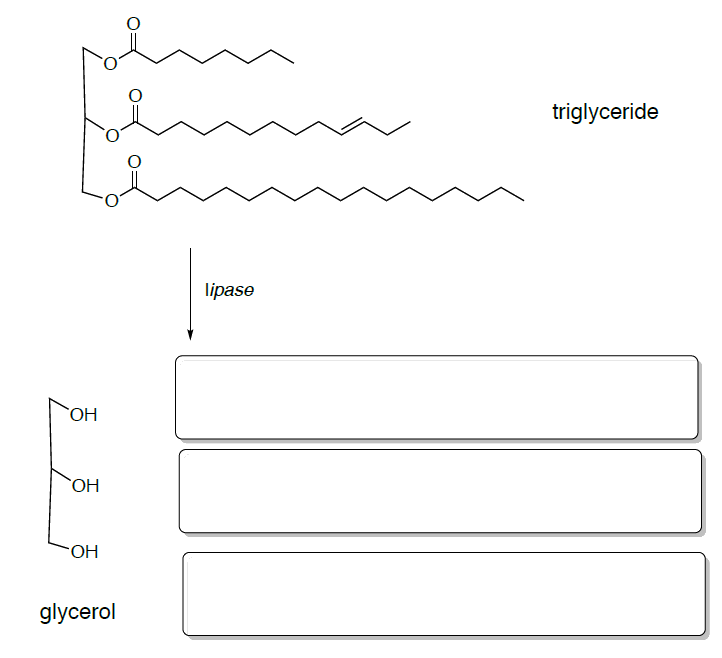
Fatty acid metabolism (b-oxidation) removes to carbons at a time to each of these fatty acids.
- Draw a couple of shorter chain fatty acids.
Esterases are often present that can turn these shorter chain fatty acids into methyl esters.
- Draw a couple of possible short chain methyl esters.
These are smelly and flavorful
Maturation of Cheese
Propionic Acid Fermentation: Flavors
Propionibacterium species are facultative anaerobes that can ferment sugars (glucose or lactose) into propionic acid. This process creates aroma and flavors found in Swiss cheeses.
A facultative anaerobe is an organism that: (choose the correct definition)
- makes ATP by aerobic respiration if oxygen is present but is capable of switching to fermentation or anaerobic respiration if oxygen is absent.
- cannot make ATP in the absence of oxygen and will die in the absence of oxygen.
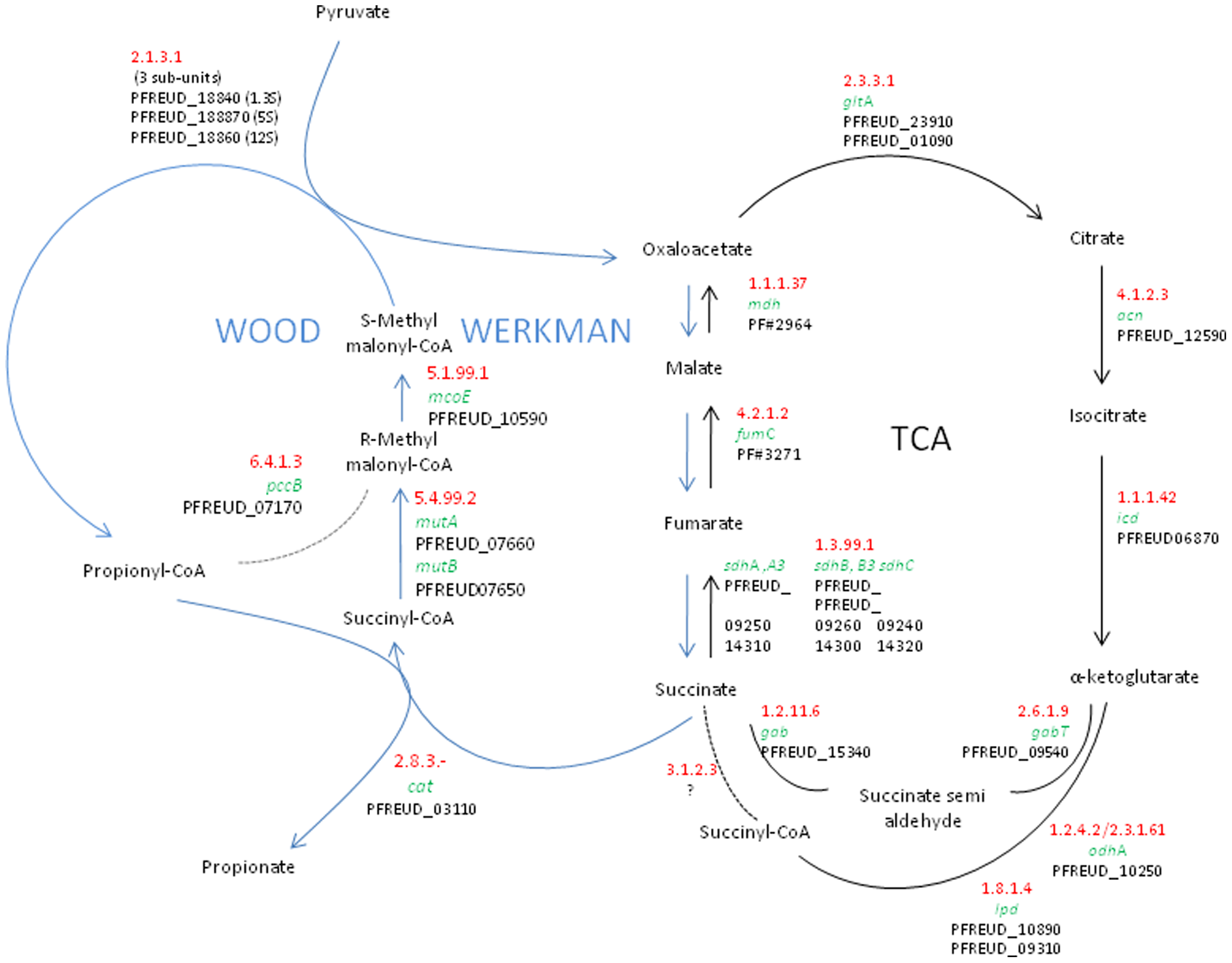
This process hijacks a part of the TCA Cycle.
- On the diagram above, circle or highlight the structures that are used in the TCA cycle.
- This pathway will function when the TCA cycle is [ on / off ] due to ____________.
- How does this pathway help the bacteria regenerate NAD+
Propionic Acid Fermentation: Flavors
A key step in the Wood-Werkman Pathway is to transfer a carboxyl group from methylmalonyl CoA to pyruvate to form propionyl CoA and oxaloacetate.
This mechanism utilizes vitamin B12 (biotin).
Draw the arrows for the decarboxylation of methylmalonyl CoA:
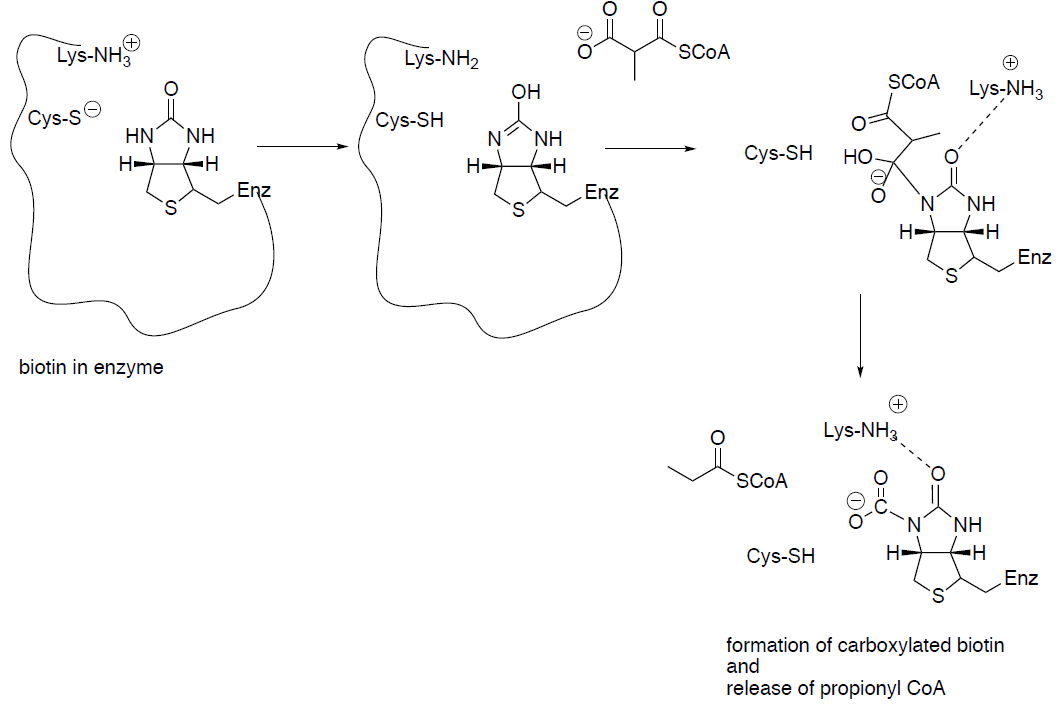
Process continues with the carboxylated biotin and the enolate of pyruvate.
Draw the enolate anion of pyruvate. Is this a nucleophile or an electrophile?
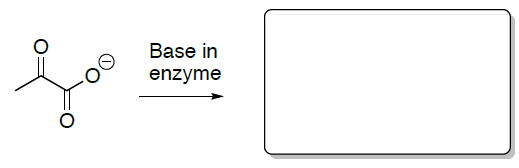
As this step continues with the carboxylated biotin and the enolate of pyruvate to form oxaloacetate.
Draw the arrows for this conversion.
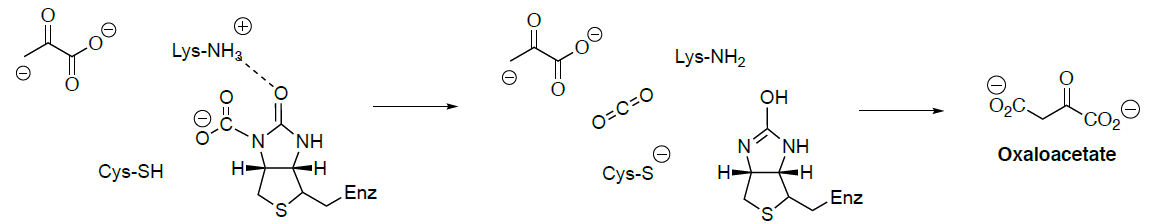
Now that you have made propionyl CoA. How is it converted to propionic acid?
Draw arrows for this trans-thioesterification process. Be sure to include a tetrahedral intermediate.
Extra Questions
- Curdling the milk is not the bacterium's only role in cheese production. The lactic acid produced by the bacterium lowers the pH of the product and preserves it from the growth by unwanted bacteria and molds while other metabolic products and enzymes produced by Lactococcus lactis contribute to the subtle aromas and flavors that distinguish different cheeses.
- Look up other chemical side products created by this bacterium and what “flavors” are imparted. (More covered in Yogurt Section!)
- A deficiency of the lactase enzyme in the small intestine gives rise to lactose intolerance, which is found frequently in most populations outside of northern Europe who are past the infant age.
If lactose is not cleaved, it cannot be absorbed, so it travels to the large intestine. Many of the bacteria found there have the capacity to metabolize lactose, which they will happily convert to acids and gas.- Would someone who is lactose intolerant be able to eat cheese? Why or why not?
Sources
D. H. Hettinga and G. W. Reinbold, THE PROPIONIC-ACID BACTERIA–A REVIEW. Journal of Milk and Food Technology, 1972, 35 (6), 358-372.


