Derivatization
- Page ID
- 160621
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Consider the analysis of alcohol in blood you studied earlier in this module. It was determined that a good choice of analytical method was headspace gas chromatography (HS-GC).
Q1. List two or three of the reasons that HS-GC was an ideal method for the quantification of ethanol in the complex sample matrix of blood.
Though not discussed in this module, the basics of gas chromatography can be found in the learning module Environmental Analysis – Lake Nakuru Flamingos: Pesticides1, with additional information available in the online textbook Analytical Chemistry 2.1.2 In general, substances in a mixture are volatilized in a heated injector port and swept onto a capillary column using an inert carrier gas like helium, where the components are separated from one another by taking advantage of differing physical interactions (structure dependent) with the column material and then individually passed through a detector, which produces an electrical signal proportional to the concentration of the individual component. A simplified diagram of a GC is shown below.

Figure 1. Block diagram of a gas chromatograph. 3
In a typical GC analysis of ethanol, the choice of detector is frequently a flame ionization detector (FID), which oxidizes the sample in an air/hydrogen flame to produce cations that are then attracted to a cathode where a current is measured. This type of detector responds to certain functional groups present on the measured species, in this case the alcohol moiety.4
Q2. Consider the methods of analysis you are familiar with or may have used in the past. For each of the methods, describe the physical process responsible for the production of an analytical signal.
In your consideration of various methods of analytical measurement, you probably noticed that for most, if not all the methods, the detection of analytes involved a physical response to some sort of external stimulation; for example, the absorption (or emission) of radiation (chromophore or fluorophore), or the oxidation or reduction of a functional group (electrophore).
Additional considerations in the choice of an acceptable method for a particular analyte must include such things as volatility and stability at elevated temperatures (most notably in GC), polarity and pH effects, solubility, chirality, poor chromatographic behavior, or just plain old lack of sensitivity sufficient for detection of the level of analyte present in the sample.5,6
Q3. What would you do if a molecule did not contain a chromophore, a fluorophore, or a redox active center, or lacked sufficient sensitivity at the chosen detector?
Many of you (hopefully) answered Q3 with some semblance of a response that included, simply stated, “change the molecule in such a way as to allow its detection”. This process whereby a molecule is chemically altered to enhance its detection by a particular analytical method is called derivatization.7
Case Study: Glyphosate (aka “Roundup”)
Glyphosate (N-(phosphonomethyl) glycine) is the most widely used herbicide in the world, with nearly 0.5 pounds/acre applied on cropland worldwide in 2014 (0.8 lb/acre in US).8,9 It was classified as a “probable human carcinogen” by the International Agency for Research on Cancer in 2015, with a “safe” level for human exposure set at 1.75 mg/kg/day by EPA.9 The structure of glyphosate is shown below.
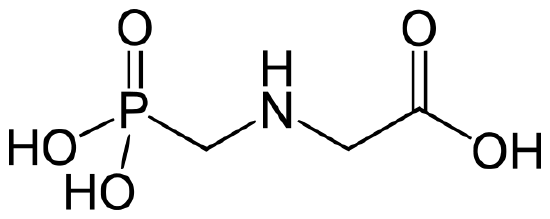
Figure 2. Structure of Glyphosate.10
Q4. The physical properties of glyphosate are listed in Table 1. Analyze the structure of glyphosate in Figure 2, along with the physical properties given in Table 1 to predict some of the considerations that must be made when choosing an analytical method for the compound dissolved in surface water.
Table 1. Physical Properties of Glyphosate.11,12
|
Molecular Weight |
169.07 |
|
Physical form |
White solid, Zwitterion structure |
|
Melting Point |
184 oC (decomposes 187 oC) |
|
Density |
1.705 (pH = 1.9, 20 oC) |
|
Dissociation Constants |
pKa1,2 = 2.0, 2.6 (phosphate); pKa3 = 5.6 (amine); pKa4 = 10.6 (carboxylic acid) |
|
log Kow |
-3.40 |
|
Solubilities |
10.5 g/L in water (pH = 1.9, 20 oC); practically insoluble in organic solvents |
Analysis of the structure and physical properties of glyphosate should lead to some generalizations as to its amenability to direct chromatographic or spectroscopic determination. It turns out that the analysis of glyphosate is complicated by the following factors (among others).8,13
- poor solubility in organic solvents, making it difficult to extract from water samples, in which it is quite soluble
- high polarity
- lack of volatility
- absence of chromophore, fluorophore, or electrophore
- generally poor chromatographic behavior (bad peak shape, adsorption, etc)
A combination of all these factors in a single molecule makes glyphosate the poster child for derivatization! Specific methods of analysis for glyphosate have been reviewed.8,13 The remaining discussion here will be of a general nature, and will deal with commonly used methods of chemical derivatization for analytical measurements that involve chromatography, including many that are applicable to glyphosate.
Common Methods of Derivatization
Gas Chromatography. Derivatization is generally necessary in the analysis by GC of compounds that exhibit low volatility, have poor thermal stability, contain “active” groups that can lead to loss of sample to intermolecular hydrogen bonding or adsorption on the inlet or column, and/or demonstrate poor sensitivity at the detector. Functional groups that are commonly derivatized for GC analysis include alcohols, carboxylic acids, amines, and sulfhydryls. The three most widely used methods of derivatization in GC are silylation, acylation, and alkylation.14
Silylation. In this reaction, active hydrogens are displaced by a silyl group, most often tetramethylsilane (TMS). The general reaction scheme is illustrated for TMS reacting with an alcohol below.
\[\ce{R-OH + (CH3)3SiX → R-O-Si(CH3)3 + HX}\nonumber\]
The reaction is applicable to active hydrogens from acid, alcohol, thiol, amine, and amide groups.6 The resulting derivatives have increased thermal and chemical stability, and increased volatility.15 Further, the presence of the silyl group improves the response in mass spectral analysis, supplying an additional characteristic fragments in the mass spectrum.15,16
Q5. The form of silane most commonly used for GC derivatization is trimethyl-chlorosilane, (CH3)3SiCl. What side reaction might you need to protect your reagent against in normal use?
Q6. Derivatization for chromatography can be performed either pre-column or post-column (prior to the detector). Which technique do you expect to be preferable for GC analysis? Explain.
In addition to trimethylchlorosilane (TMCS), a large number of silylation reagents exist that have differing reactivities and specificities. Many classes of compounds, like steroids, may possess more than one type of functional group that requires derivatization by more than one reagent.14 More information on the specifics of silylation can be found in review articles6,16-17 or from vendors like Sigma-Aldrich who have a large collection of application notes and product information available on their web sites.15,18
Acylation. In this process, compounds with active hydrogens (OH, SH, NH) are reacted with carboxylic acid or derivatives of carboxylic acids to form esters, thioesters, and amides, respectively.6 The general reaction for the acylation of an alcohol by an acid halide to form an ester is shown below.
\[\ce{R-C}\textrm{(:O)}\ce{-X + R’-OH → R-C}\textrm{(:O)}\ce{-O-R’ + HX}\nonumber\]
In addition to acid halides, acylation reagents include acid anhydrides and reactive acyl derivatives like acylated imidizoles and perfluorinated acyls.15 As was the case for silylation, acylation produces derivatized species that are more volatile, generally less polar, and less prone to adsorption than the original compound. Derivatization with fluorinated acyl groups leads to enhanced detectability at the electron capture (EC) detector for GC, as well as increased structural information from mass spectrometric detection.16 In general, acylated derivatives are more stable than their silylated counterparts.15
Popular acylation reagents include acetic anhydride (AA) trifluoroacetic acid (TFA), trifluoroacetic anhydride (TFAA), trifluoroactylimidazole (TFAI), pentafluoropropionic anhydride (PFAA), and N-methylbistrifluoroacetamide (MBTFA).6 Reagents useful for the derivatization of carbohydrates, amino acids, and drugs of abuse are also commercially available. The reader is once again directed to vendor web sites for information specific to a particular analysis.15,18-19
Q7. Do a Google search for 2,4,6-trichloranisole (TCA). What is it? Draw its structure, and predict whether the molecule would be amenable to derivatization by acylation. Explain your answer.
Q8. Resveratrol is an anti-oxidant commonly found in red wine. Click on the link given in reference 15, which will take you to information on derivatization reagents available on the Sigma-Aldrich website. Locate the application note for SPME analysis of resveratrol (page 26). Give the structure of resveratrol. What SPME fiber is used and what derivatization of TCA is required for analysis?
Alkylation. Derivatization by alklylation involves the replacement of active hydrogens of the type described previously with an aliphatic or aliphatic-aromatic (like benzyl) group. The most common application of the technique is the formation of esters from carboxylic acids. The esters formed in this way are less polar, more volatile and display improved chromatographic behavior than the original compound. In general, these derivatives are more stable than the corresponding silylation product.15 Esterification is most often accomplished by reaction of an alcohol with the carboxylic acid in the presence of an acid catalyst. This reaction is shown below.20
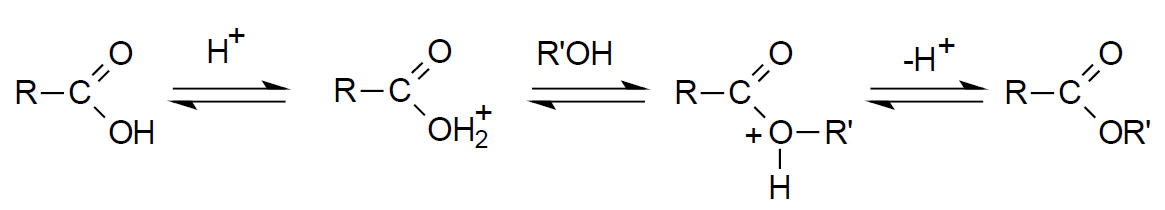
Rapid esterification (~2 min) can be accomplished at 100 oC (water bath) with BF3-methanol solution.6
A more general alkylating agent is N,N-dimethylformamide dimethylacetal (DMFDA), whose structure is given in Figure 3.
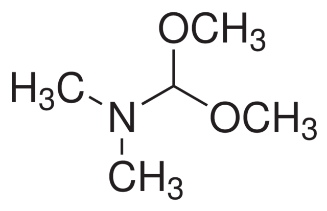
Figure 3. Structure of DMFDA.21
In addition to carboxylic acids, DMFDA rapidly reacts with amines, phenols, thiols, and amino acids to give the products in Table 2.6
|
Compound |
Derivative |
|---|---|
|
R-COOH |
R-COOCH3 |
|
R-NH2 |
R-NH-(CH2)2-N(CH3)2 |
|
R-OH |
R-O-CH3 |
|
R-SH |
R-S-CH3 |
|
-O-C(O)-CH(R)-NH3+ |
CH3-O-C(O)-CH(R)-NH-(CH2)2-N(CH3)2 |
Table 2. Reaction Products for DMFDA Derivatization.
Derivatization with DMF-Acetals containing longer side chains (between 2 and 4 carbons) are also possible, with the added benefit of controlling analyte retention times.16
As with previous derivatizations, the addition of halogens in the alkylation reaction will enhance the response at the EC detector for derivatized analyte. One commonly used reagent for this purpose is pentafluorobenzyl bromide (PFB-Br), which is capable of adding the PFB group to carboxylic acids, alcohols, sulfonamides, and thiols.6
Liquid Chromatography. High performance liquid chromatography (HPLC) is preferred for separations involving chemical species that do not exhibit sufficient volatility or stability to allow the use of gas chromatography (GC). Applications of HPLC routinely include drug analysis, measurement of biologically important species like amino acids, and pesticide analysis, among others. Often these analytes lack a necessary structural component that makes them easily detectable using common techniques of HPLC detection like spectrophotometry, fluorimetry, or electrochemistry. As in GC analysis, methods to improve the detectability of a target analyte by reaction with a suitable derivatizing agent are available for HPLC. In addition to detectability, the derivatization of polar compounds can increase their retention in reverse-phase HPLC by making the derivatized product more hydrophobic.15
Q9. Consider the HPLC analysis of glyphosate in groundwater utilizing spectrophotometric detection. Due to the lack of a chromophore or fluorophore in the structure of the herbicide, it is necessary to perform a derivatization reaction before the modified analyte can be detected (see Figure 4). Speculate on the stages in the sample prep/analysis where a conversion can be achieved. Are there advantages and disadvantages that you recognize with each of your choices?
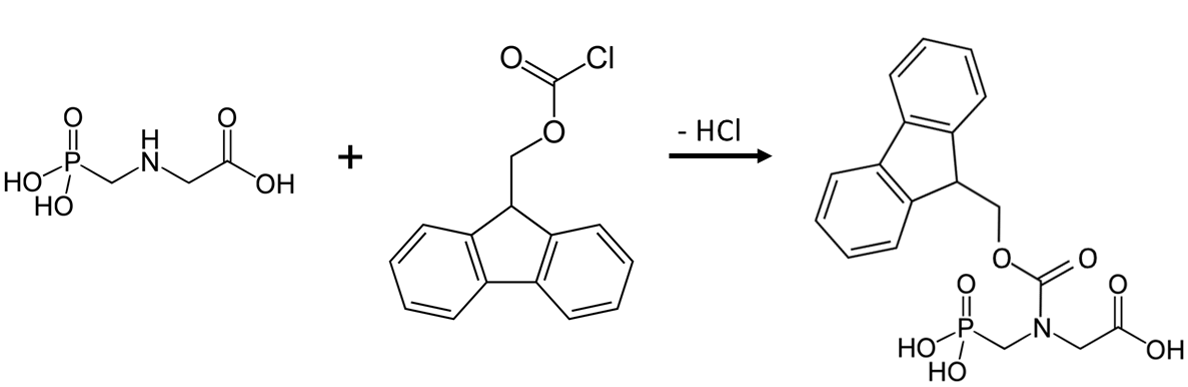
Figure 4. Reaction of Glyphosate with 9-Fluorenyl Methoxycarbonyl Chloride (FMOC-Cl)13
Q10. Identify the structural characteristic(s) of the derivatized glyphosate that now allow for spectrophotometric quantification. Specifically, how would you now expect to quantify the molecule with the maximum amount of sensitivity? Explain your answer.
While derivatization in GC occurs nearly always pre-injection, derivatization reactions in HPLC are generally completed either pre-column, where sample and reagent are mixed prior to injection, or post-column, where separation of the sample components is achieved prior to reaction with derivatizing agent.
With appropriate instrumentation, both techniques can be carried out in an on-line mode, where the addition of reagent and subsequent mixing is performed by the instrument.22 In some instances, pre-column derivatization is best done off-line, allowing for more complicated sample manipulations, and removal of excess reagent or interferents prior to injection. Often the choice is dependent upon the speed with which a derivatization reaction can be made to occur, the stability of the derivatized product, or whether automation is available to accomplish the reaction.23
Important distinctions between pre- and post-column derivatization are summarized below.22
- Pre-column
- no restrictions on reaction conditions
- quantities of derivatizing agents can be minimized
- provides generally better analyte sensitivity due to lower backgrounds
- requires reaction products that are stable over time
- Post-column
- reaction must be rapid and compatible with eluent
- derivatization reagent mixed with chromatographically pure analyte after elution
- unstable products can be analyzed immediately
- constant flow of reagent stream requires large quantities of derivatizing agent
Derivatization for HPLC most often involves the introduction of a “tag” to an analyte molecule that imparts to the derivatized product a physical property lacking in the underivatized analyte, which can then be exploited in the detection process. Three of the most important functional groups in derivatization for HPLC are chromophores, fluorophores (or a species capable of forming a fluorophore with the analyte), and redox-active centers.23
Classes of molecules that frequently require derivatization to allow detection at desired concentration levels include aliphatic amines, carboxylic acids, and alcohols/thiols.6 For HPLC, derivatization reactions can be easily accomplished in either aqueous or non-aqueous solvent, leading to multitudinous methods for both pre- and post-column reactions.6 Review articles are available that provide detailed coverage of these methods.5-6, 22-24 Some of the more frequently encountered methods will be presented here.
UV-Visible Detection. Though the most widely utilized type of detection for HPLC, UV-Vis detection lacks the necessary sensitivity/selectivity for many analytes. Derivatization generally involves the introduction of highly conjugated aromatic moieties to the analyte, giving the product high absorptivity and improved detection.15
Examples of derivatizing agents commonly employed for HPLC/UV-VIS are given in Table 3.
|
Derivatizing Agent |
Group(s) Derivatized |
|---|---|
|
1-Fluoro-2,4-Dinitrobenzene (FDNB) |
RNH2, RNHR’, R-OH |
|
4-N,N-Dimethylaminoazobenzene-4’-Sulfonyl Chloride (DABS-Cl) |
RNH2, RNHR’, R-SH, R-OH |
|
p-Bromophenacyl Bromide (BPB) |
R-COOH, R-SH |
|
Benzoyl Chloride |
RNH2, RNHR’, R-SH, R-OH |
|
9-Fluorenylmethoxycarbonyl Chloride (FMOC-Cl) |
RNH2, RNHR’, R-OH |
|
2,4-Dinitrophenylhydrazine (2,4-DNPH) |
RC(O)R’, RC(O)H |
Table 3. Common Derivatizing Agents for HPLC/UV-VIS Analysis.
Specific applications can be used to illustrate the usefulness of these reagents.
FDNB reacts with primary and secondary amines, and aliphatic hydroxyl groups.23 For example, the tricyclic amine memantine hydrochloride, useful in the treatment of Parkinson’s disease and other movement disorders, was analyzed in finished dosage forms following pre-column derivatization with FDNB. UV detection of the product was accomplished at 360 nm.25
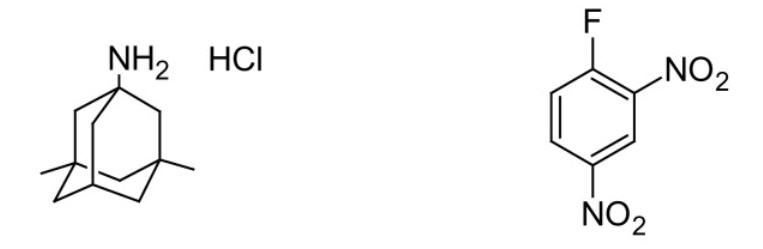
Figure 5. Structures of Memantine hydrochloride (left) and FDNB (right).25
Q11. What do you predict the reaction product to be for the reaction between memantine HCl and FDNB? Draw its structure.
The sulfonyl group in DABS-Cl, whose structure is shown in Figure 6, can react with primary and secondary amines, alcohols, and thiols to form derivatives with strong visible absorption between 425 and 468 nm.24 Recently, the complete amino acid analysis of octreotide, used clinically to treat endocrine and carcinoid tumors, among other things, was accomplished using DABS-Cl derivatization followed by visible detection at 436 nm.26
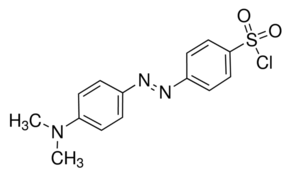
Figure 6. Structure of DABS-Cl.
Q12. Do an internet search for octreotide and locate its structure. Considering only the primary amine sites on the molecule, predict how many sites will be derivatized with DABS-Cl. Next, using “R” as the rest of the molecule, show the structure of the reaction product of DABS-Cl and a single primary amine.
Aromatic halides like p-bromophenacyl bromide (BPB) are effective derivatization agents for carboxylic acids, producing strongly absorbing phenacyl ester derivatives.15 Pre-column derivatization of fatty acid with BPB in the presence of crown ether to aid in product extraction into non-aqueous solvent allowed detectability limits of 50 ng of derivatized acid containing 20 carbons. UV detection was carried out at 254 nm.27
BPB has been used to derivatize the thiol moiety of tiopronin, a drug used to prevent kidney stones and in the treatment of rheumatoid arthritis, in blood plasma. The derivatization reaction is given in Figure 7. UV detection was done at 236 nm, with a reported limit of detection for tiopronin in plasma of 12 ng/mL.28
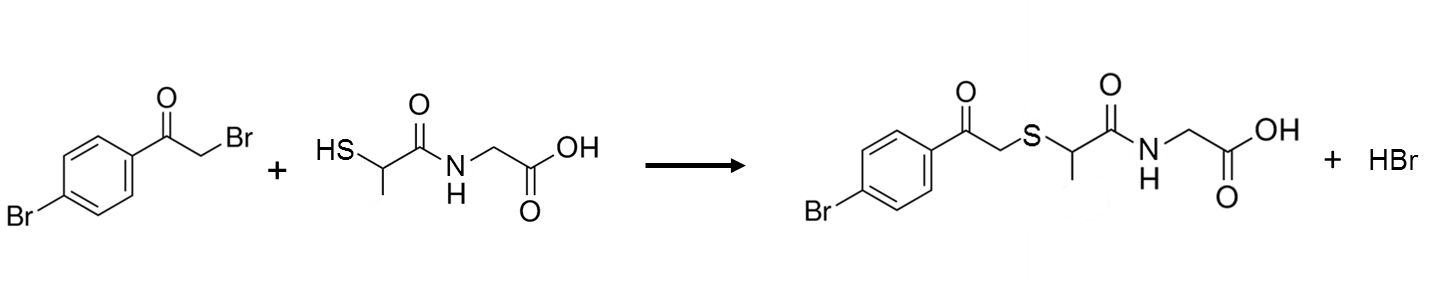
Q13. BioRad maintains a free database of spectra, where you can obtain the UV spectra of 4-bromophenacyl bromide (https://spectrabase.com/), shown below. Speculate as to why the derivatized fatty acid and derivatized tiopronin above were detected at 254 and 236 nm.
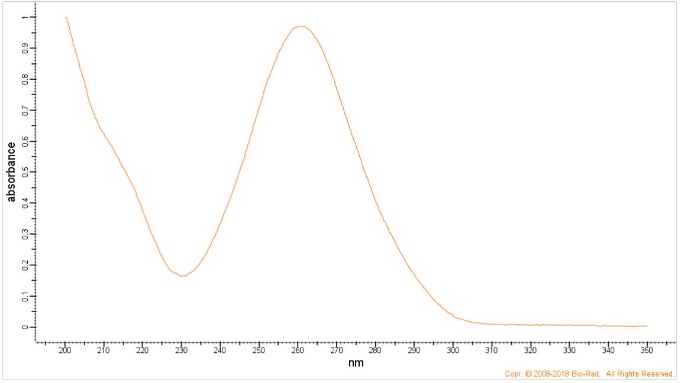
Figure 8. UV Spectrum of 4-Bromophenacyl Bromide.
Hydroxy compounds, including alcohols and phenols, carbohydrates and steroids, can be derivatized by reaction with acyl chlorides like benzoyl chloride that convert the hydroxyl group into an ester.15 Additionally, this reagent can react with primary and secondary amines, thiols, and phenols to introduce a spectroscopically useful phenyl group. A recent report demonstrated the utility of benzoyl chloride in the derivatization of 70 neurologically relevant compounds, including catecholamines and their metabolites prior to analysis by HPLC/MS/MS.29
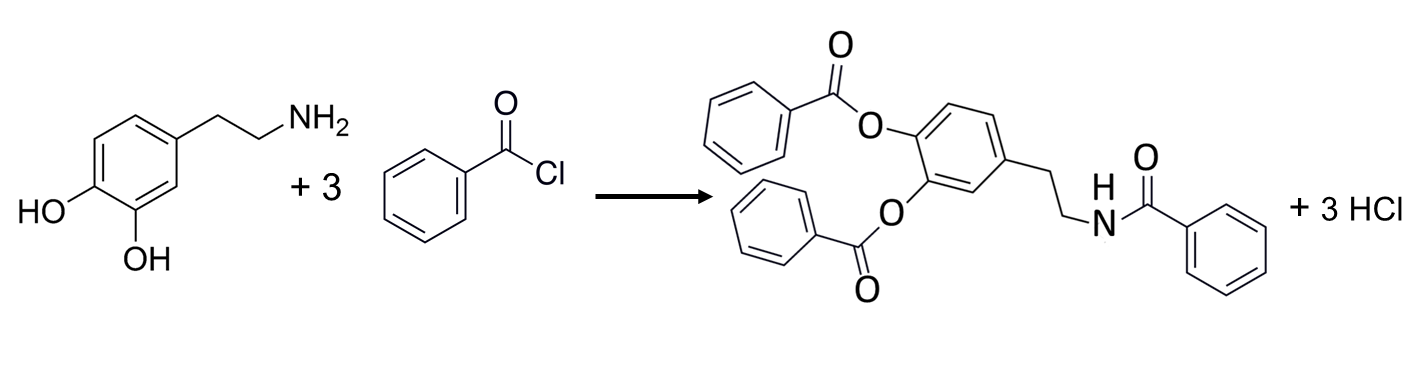
Figure 9. Derivatization of Dopamine (left) with Benzoyl Chloride.
FMOC-Cl, to which you were introduced earlier in this module, has been used to derivatize primary, secondary, and to some extent tertiary amines, as well as for some alcohols. It has been shown to be useful for enhancement of both UV detection (at 265 nm) and fluorescence detection (excitation at 265 nm and emission at 340 nm).24 Its reaction with glyphosate was seen in Figure 4. FMOC-Cl has been widely used for analysis of amino acids. It can be used in conjunction with o-phthalaldehyde (OPA), selective for primary amines, to doubly derivatize amino acids and biogenic amines in biological tissues.31 The structures of OPA and FMOC-Cl are given in Figure 10.
Figure 10. Structures of OPA (left) and FMOC-Cl (right).
Q14. Look up the structures of putrescine and spermidine, and determine how they differ. Explain what advantage double-derivatization might offer in the identification of these molecules in a mixture.
2,4-DNPH is one of the most widely used derivatizing agents in analytical analysis, converting carbonyl compounds to their corresponding 2,4-dintrophenylhydrazone derivatives.32 The general reaction scheme is given in Figure 11.
Figure 11. Reaction of 2,4-DNPH (left) with Carbonyl to Form DNP-hydrazone.
Important applications of this method can be found in the analysis of carbonyl compounds arising from motor vehicles and industrial emissions, as well as “sick building syndrome” involving airborne volatile molecules like formaldehyde.33 2.4-DNPH-coated silica gel cartridges have been used in the collection of formaldehyde from ambient air, with extraction into organic solvent and detection at 366 nm.34 A procedure for the analysis of 20 carbonyl compounds in aqueous, soil, waste, and stack samples by UV absorption at 360 nm following 2,4-DNPH derivatization can be found in EPA Method 8315A.35
Q15. Use the link in Reference 35 to obtain a copy of EPA Method 8315A. Outline the procedure necessary to quantify carbonyl compounds (other than formaldehyde) in aqueous samples. Next, determine how the MDL values for hexanal and decanal isolated from solid samples differs from that for liquid samples.
Fluorescence Detection (FD). Owing to low cost and high availability in many laboratories, UV-VIS detection is the most widely used method for the determination of derivatization products in HPLC.28 Fluorescence detection, which is less commonly available, offers lower detection limits and wider linear range than does UV-VIS.15 The technique has better inherent specificity than absorption alone since both excitation and emission wavelengths are controlled. Emission is generally measured at 90o to exciting radiation to minimize stray light.36
Many of the derivatization agents for enhanced UV-VIS detection in HPLC form products that are also amenable to fluorescence detection. Table 4 lists some of the more commonly used compounds for the formation of fluorescent derivatives.
|
Group(s) Derivatized |
|
|---|---|
|
o-Phthalaldehyde, OPA |
RNH2, RNHR’ |
|
Dimethoxyanthracene sulfonate, DMAS |
RNH2, RNHR’, R3N |
|
2,3-Naphthalene dialdehyde, NDA |
RNH2, RNHR’ |
|
Monobromobimane, MBB |
R-SH |
|
9-Fluorenylmethoxycarbonyl chloride, FMOC-Cl |
RNH2, RNHR’, R3N , -OH |
|
5-Dimethyl amino naphthalene-1-sulphonyl chloride (Dansyl chloride), DNS-Cl |
RNH2, RNHR’, \(\Phi\)-OH |
|
5-(Dimethylamino)-1-naphthalenesulfonic hydrazide, DH (Dansyl hydrazine) |
RC(O)R’, RC(O)H, RCOOH |
Table 4. Common Derivatizing Agents for HPLC/FL Analysis.
Examples of methods utilizing these fluorescence derivatizing agents are given below.
o-Phthaladehyde. Perhaps the most commonly used derivatizing agent in the production of fluorescent products is o-phthalaldehyde (OPA). OPA reacts with primary amines and amino acids in the presence of thiol or sulfite to produce isoindole derivatives.23 Secondary amines can be also derivatized following the addition of NaOCl.
Gabapentin has been used to treat epilepsy and is known to increase the production of \(\gamma\)-aminobutyric acid (GABA) in the brain.37 A methanolic solution of OPA/mercaptoethanol was used to derivatize gabapentin in blood plasma after clean-up on a C18 SPE column. Excitation frequency was 350 nm, with emission monitored at 450 nm. Quantitation of the drug was possible down to a level of 0.3 mg/L, with the therapeutic range reported to be 2-20 mg/L. The formation of the fluorescent derivative of gabapentin is shown below.
Figure 12. Reaction of o-Phthalaldehyde with Gabapentin.
Q16. What properties are associated with fluorescent molecules?
9,10-Dimethoxy-2-Anthracene Sulfonate. Dimethoxy anthracene sulfonate (DMAS) is capable of forming fluorescent ion pairs with amines and has been successfully used in the quantification of drug substances such as chloropheniramine and ephedrine, which contain teritary amine groups.6 The spectrofluorometric determination of the β-blockers arotinolol, atenolol and labetalol derivatized with DMAS has been reported by Abdine, et al.38 The formation in acidic medium of the fluorescent charge transfer complex between DMAS and arotinolol, which has a 2:1 stoichiometry, is shown below.
Figure 13. Reaction of Arotinolol with Dimethoxyanthracene Sulfonate.
The ion pairs for each of the β-blockers were extracted from tablets and from serum with chloroform, excited at 385 nm and measured at 452 nm. The reported LOD for arotinolol was 0.12 μg/mL.
Q17. What is a charge transfer complex?
2,3-Naphthalene Dialdehyde. While OPA is a very popular choice as a fluorescent tag for amines and amino acids, its use suffers from several drawbacks, including short derivative lifetimes and unfavorable side reactions with imidazole-containing compounds like histamine. The naphthalene derivative of OPA, 2,3-naphthalene dialdehyde (NDA), has been shown to ameliorate these problems, leading to more stable derivatives and more sensitive detection for many applications.39
Li, et al. studied the reaction of glutathione with NDA and two newly synthesized derivatizing agents containing methoxy and fluorine groups respectively at the 6-position of the naphthalene ring of NDA.40 Of the three probes, NDA showed the best results with glutathione, with a calculated detection limit of 6.4 x 10-8 M. The formation of the fluorescent derivative of glutathione (GSH) with NDA is shown below.
Figure 14. Reaction of Glutathione with 2,3-Naphthalene Dialdehyde.
Maximum excitation of the derivatized product was observed at 450 nm, with detection being done at an emission wavelength of 531 nm.40 The probes were then used in fluorescence microscopy measurements of GSH in live cells.
Q18. Naphthalene, the base compound in the formation of NDA, is one of a class of compounds called polycyclic aromatic hydrocarbons (PAH) which are known or suspected carcinogens. An interesting analysis scheme for these compounds involves the use of cyclodextrins to enhance the energy transfer between PAH and the strong fluorophore acceptors BODIPY and Rhodamine 6G. Use the supplied link to access the report, find the structures of the two fluorophore acceptors, and the mechanism by which cyclodextrin compounds contribute to the analysis (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4376008/ ).
Monobromobimane. MBB reacts with compounds containing reactive sulfur or thiol groups, including H2S, glutathione, proteins, and nucleotides.41 While the probe itself has little fluorescence, highly fluorescent and extremely stable thioether derivatives are formed quickly at room temperature.
Shen, et al. have reported the sensitive measurement of hydrogen sulfide in plasma by HPLC following derivatization with MBB.42 H2S is produced in mammalian tissue during the metabolism of cysteine. It is involved as a gaseous mediator in the cardiovascular and other systems, and is linked to a number of disorders, including Alzheimer's disease. In this study, plasma samples were incubated with MBB in Tris buffer at pH 9.5 for 30 minutes, and the fluorescent product quantified following RP-HPLC at an excitation wavelength of 390 nm and an emission wavelength of 475 nm. The detection limit for hydrogen sulfide was found to be 2 μM. The
Figure 15. Reaction of Monobromobimane with Hydrogen Sulfide.
Q19. Locate the structure of cysteinylglycine, and its biological function. What product would you expect in the reaction of cysteinylglycine with MBB?
9-Fluorenylmethoxycarbonyl chloride. As was mentioned in the UV-VIS section, FMOC is an effective derivatizing agent for primary, secondary, and tertiary amines, as well as some alcohols. Its reaction with glyphosate was given in Figure 4.
Q20. Access EPA Method 547 for glyphosate at https://www.o2si.com/docs/epa-method-547.pdf, and give a short summary of the method. What is the HPLC column specified in the method, and what is the reported MDL for glyphosate in ground water?
The tricyclic antidepressant drug, nortriptyline (NT), a non-selective serotonin uptake inhibitor, was quantified in plasma samples by FMOC-Cl derivatization.43 The reaction was carried out at room temperature for 30 minutes, and the product was stable for at least 48 hours. A wide calibration range between 5-5000 ng/mL was obtained, and an LOD of 2 ng/mL was reported. Excitation wavelength was 260 nm with emission detection at 310 nm. The derivatization scheme for nortriptyline is shown below.
Figure 16. Reaction of 9-Fluorenylmethoxycarbonyl Chloride with Nortriptyline.
5-Dimethyl amino naphthalene-1-sulphonyl chloride. Dansyl chloride (DNS-Cl), like OPA, has found widespread use in the analysis of amino acids at very low levels, in the nanomole to picomole range.44 An advantage of DNS-Cl over OPA is that the derivatized products are more stable, and give better results at the lower concentrations.
DNS-Cl can be successfully applied to the derivatization of polyamines to form fluorescent products for HPLC analysis. Molin-Legua, et al. describe the use of DNS derivatization in the quantification of putrescine, cadaverine, spermidine, and spermine in urine following purification by solid phase extraction (SPE).45 After pre-concentration of the analytes of interest on the SPE packing, DNS-Cl reagent in buffer at pH 9.5 was introduced to the column where the derivatization reaction was allowed to take place for 30 minutes at room temperature. The fluorescent derivatives were desorbed using acetonitrile and introduced into the HPLC. Excitation was accomplished at 252 nm, while emission was measured at 500 nm. Detection limits for the four polyamines in urine was 2 ng/mL. The reaction scheme is illustrated below for putrescine.
Figure 17. Reaction of Putrescine with 5-Dimethyl Amino Naphthalene-1-Sulphonyl Chloride.
5-(Dimethylamino)-1-naphthalenesulfonic hydrazide. Dansyl hydrazine (DH), reacts with ketones (including ketosteroids), aldehydes, and reducing sugars to form intensely fluorescent hydrozones.46 The reagent itself is highly fluorescent and can lead to interference if not removed from the reaction mixture prior to analysis.
2,5-hexanedione (2,5-HD) is a metabolite of hexane exposure in mammals. It is an active neurotoxin and has been related to testicular cancer in mice. The presence in urine can signal hexane exposure, often as a result of workplace use, gasoline, or from voluntary “sniffing” of solvents.47-48
Maestri, et al. have reported a sensitive method for determining 2,5-HD in urine by HPLC after derivatization with dansyl hydrazine.49 Urine samples were purified with C18 cartridges prior to derivatization. The hydazone derivative of 2,5-HD was detected by fluorescence, with an excitation wavelength of 340 nm and an emission wavelength of 525 nm. The reported detection limit for 2,5-HD in urine was 5 μg/L. The reaction between 2,5-HD and DH is shown below.
Figure 18. Reaction of 2,5-Hexanedione with 5-(Dimethylamino)-1-Naphthalenesulfonic Hydrazide.
Q21. What is a ketosteroid? Give the reaction product of a typical 17-ketosteroid with DH.
Electrochemical Detection (ECD). Electrochemical detection for HPLC first came into prominence in the 1970’s as the detector of choice in measurement of endogenous catecholamines and indoleamines, providing extremely low levels of detection (down to femtomolar range) for these substances in neurochemical analysis.50-53 The catechols and indoles lent themselves well to the technique, being inherently active electrochemically at relatively low applied potentials.
Electrochemically active compounds include aromatic alcohols and amines, nitroaromatics, heterocyclics, aliphatic amines, and aliphatic polyenes.54 By varying the potential applied in ECD, a certain degree of specificity can be achieved for some analytes in the presence of others.
Q22. Access the Basic Principles document on electrochemical detection at the BASi website (www.basinc.com/manuals/LC_epsilon/Principles/Basic/basic#hdv), and scroll down to the section on “Hydrodynamic Voltammograms” containing Figure 1.7. Explain in a few sentences how applied detector potential allows you to choose which analytes you wish to quantify.
Two factors limit the usefulness of electrochemical detection for the majority of analytes: 1) lack of an electroactive functional group that can be oxidized or reduced at a reasonable potential, or 2) presence of an electroactive group whose redox potential lies outside the working window of the device.55-56
Figure 19 presents approximate ranges for the oxidation/reduction potentials for many common organic functional groups.57
Figure 19. Oxidation/Reduction Potentials for Common Organic Functional Groups
(adapted from Fuchigami, Reference 57)
Q23. The structure of dopamine, a neurotransmitter in mammalian brain known to be involved in a variety of motivated behaviors, was given in Figure 9. It can be easily oxidized at a carbon electrode at potentials less than +0.5 V vs SCE. What functional group(s) would you expect to oxidize at these potentials, and what do you think is a reasonable product if the oxidation mechanism involves 2 electrons and 2 protons?
Q24. In the Basic Principles document accessed in Q22, scroll down to the section on “Background Current” containing Figure 1.14. What are the approximate potential windows, both anodic and cathodic, for routine ECD? What common sources of background current can limit the extent of the available potential window?
As was the case previously for spectroscopic detection in HPLC, the introduction of a derivatization reagent can often allow the analyst to take advantage of the enhanced sensitivities and limits of detection available in ECD for substances that are not naturally amenable to analysis by this method.54,58 Many of the same reagents used to produce fluorescent derivatives can also be used to introduce an electrophore to the molecule, though fluorescence detection is used more extensively than electrochemical detection.59
Q25. Based on the potential windows arising from background current found in Q24, which of the groups in Figure 19 would likely require derivatization to allow successful use of electrochemical detection in HPLC?
Two types of electrochemical detectors are in common use.54,60-62 In an amperometric detector, the effluent from the chromatographic system is passed over a smooth electrode surface at which the potential is controlled and the current resulting from oxidation or reduction of the analyte is measured. This type of detector is characterized by low noise and high sensitivity, but only about 5-15% of the total amount of analyte comes in contact with the electrode, which limits the limit of detection. In a coulometric detector, a high surface area porous electrode is utilized through which the column effluent flows, and at which close to 100% of the analyte is oxidized/reduced. This results in a higher signal and in many cases lowered detection limits.
The use of the amperometric detector is limited primarily to isocratic elution for HPLC as changes in eluent composition lead to changes in the observed background current. The use of the coulometric detector reduces the effect of background current changes and can be used for gradient elution.54,56
Examples of derivatization agents used in the production of electroactive products for HPLC are given in Table 5.
|
Derivatizing Agent |
Group(s) Derivatized |
|---|---|
|
o-Phthalaldehyde, OPA |
RNH2, RNHR’ |
|
2,3-Naphthalene dialdehyde, NDA |
RNH2, RNH2R’ |
|
Ferrocene derivatives |
RNH2, RNH2R’, R-COOH, R-OH , R-SH, RC(O)R’, RC(O)H |
|
1-(2,5-dihydroxyphenyl)-2-bromoethanone, 2,5-DBE |
R-COOH |
|
4-NPH |
RC(O)R’, RC(O)H |
Table 5. Common Derivatizing Agents for HPLC/ECD Analysis.
o-Phthalaldehyde. An example of the use of OPA in ECD derivatization is for histamine, which in its native form requires an applied potential of >1.2 V vs Ag/AgCl for detection. The OPA/2-mercaptoethanol derivative of histamine can be detected at a modest applied potential of +0.4 V.63
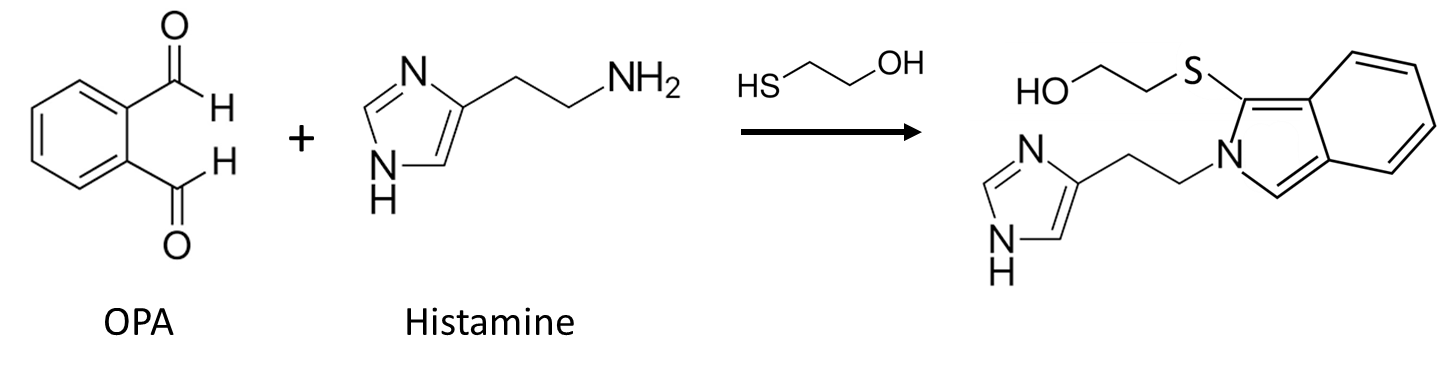
Figure 20. Reaction of o-Phthalaldehyde with Histamine.
Q26. Using the potential ranges in Figure 19 as a guide, predict which of the functional groups on OPA-derivatized histamine leads to the observable signal observed near +0.4 V vs Ag/AgCl.
Reaction times are on the order of 2 minutes for OPA, making its use as a post-column derivatizing agent possible. The reaction product is highly unstable, though the use of sulfite in place of thiol has been shown to produce a more stable derivatized product.58 Chen, et al. have reported the detection of histamine in brain diasylate after OPA/sodium sulfite derivatization using dual detectors.64 The first was poised at a potential of +0.25 V vs Ag/AgCl (where the histamine product did not oxidize) to reduce noise from other chemicals present, with a second detecting derivatized histamine at +0.55 V vs Ag/AgCl.
2,3-Naphthalenedialdehyde. Another frequently encountered fluorescent derivatizing agent, 2,3-naphthalenedialdehyde (NDA), reacts with primary amines and amino acids in the presence of cyanide ion to produce electroactive products that are detectable at ca. +0.75 V vs Ag/AgCl.58,65 Reaction times are greater than those observed for OPA (15-60 minutes), but the reaction products are more stable, making pre-column derivatization easier.23 The reaction between NDA and a primary amine in the presence of cyanide to form a cyano[f]benzoisoindole (CBI) is shown below.
NDA CBI
Figure 21. Reaction of 2,3-Naphthalenedialdehyde with Primary Amine.
Ferrocene. Ferrocene (bis-cyclopentadienyl Fe(II)) and its substituted derivatives display highly reversible electrochemical behavior. Fe(Cp)2 has an accessible redox potential in organic solvents near +0.5 V vs. SCE, with the moiety retaining its excellent electrochemical performance even when attached to more complex molecules.66 This makes it an exceptional choice as a derivatizing agent for HPLC-ECD.
Figure 22. Structure of Ferrocene.
Q27. Predict what might happen to the redox potential of ferrocene as the cyclopentadienyl groups are modified by the addition of different functional groups like methyl, alkylamine, or carboxylic acid. Explain.
Ferrocene-based derivatizing agents have been applied most widely to the analysis of amine containing compounds including amino acids, peptides and proteins.56 By altering the reactive groups on the ferrocene, derivatives have been formed with oxidation potentials generally in the range of +0.20 to +0.60 V vs Ag/AgCl.56,59 Ferrocene carboxaldehyde and ferrocene carboxylic acid chloride (FAC) have been used to derivatize primary amines, with FAC also reacting with secondary amines to form stable products that are easily oxidized.65,67 The reaction between FAC and amino acid to form electroactive product is shown below.
Figure 23. Reaction of Ferrocene Carboxylic Acid Chloride with Amino Acid.
In addition to amines, ferrocene containing reagents have been used to derivatize alcohols, thiols, ketones, aldehydes, and carboxylic acids for analysis by HPLC-ECD resulting frequently in limits of detection in the femtomolar range.56,59,65 The sulfhydryl group of cysteine can be selectively reacted with ferrocene derivatives, as shown below for the reaction of glutathione (GSH) with ferrocenyl ethylmaleimide (FEMI).68
Figure 24. Reaction of Glutathione with Ferrocenyl Ethylmaleimide.
Other derivatizing agents. Carboxylic acids have been derivatized using substituted phenacyl bromides, as illustrated below for the reaction between 1-(2,5-dihydroxyphenyl)-2-bromoethanone (2,5-DBE) and quinoxaline-2-carboxylic acid (QCA). The resulting ester derivatives were oxidized between 0.4 – 0.6 V vs Ag/AgCl.69
Figure 25. Reaction of 1-(2,5-Dihydroxyphenyl)-2-Bromoethanone with Quinoxaline-2-Carboxylic Acid.
Alcohols and ketones can be derivatized using aromatic nitro-derivatives.63 As an example, Shimada reported the electrochemical detection of the hydrazone derivative of 17-ketosteroids at +0.8 V vs Ag/AgCl following reaction with 4-nitrophenylhydrazine (NPH).70 This reaction is shown below for the derivatization of androsterone (AND).
Figure 26. Reaction of 4-Nitrophenylhydrazine with Androsterone.
Q28. The use of 4-nitrophenylhydrazine introduces more than one electrochemical active functionality to androsterone in the reaction from Figure 26. What two groups in the derivatized molecule would you would expect to be addressable via ECD? Approximately what detector potential would be needed for each? Which group do you think is reacting at +0.8 V vs Ag/AgCl?
Detailed derivatization procedures, conveniently organized by both functional group and derivatizing agent, are collected in Handbook of Derivatization Reactions for HPLC, edited by Lunn and Hellwig.71 In addition, tables of derivatizing agents and citations to primary literature can be found in references 54, 56, 59, and 65.
References
- Bullen, H.A., et al. Environmental Analysis – Lake Nakuru Flamingos: Pesticides; Analytical Sciences Digital Library, 2013. community.asdlib.org/activelearningmaterials/environmental-analysis-lake-nakuru-flamingos/ (accessed May 23, 2017).
- Harvey, D. T., Analytical Chemistry 2.1, A Free Digital Resource for Analytical Chemistry, 2016. http://dpuadweb.depauw.edu/harvey_web/eTextProject/version_2.1.html (accessed May 23, 2017).
- http://elte.prompt.hu/sites/default/files/tananyagok/AtmosphericChemistry/ch15s02.html (accessed May 23, 2017).
- Kinton, V. R.; Pfannkoch, E. A.; Whitecavage, J. A. Blood Alcohol Analysis Using An Automated Static Headspace Method. Gerstel, Inc. USA. www.gerstelus.com/media/1103/2005-01_mps2_hs-blood-alcohol.pdf (accessed May 23, 2017).
- Rosenfeld, J. Chemical Derivatizations in Analytical Extractions. In Handbook of Sample Preparation; Pawliszyn, J., and Lord, H., Eds.; John Wiley & Sons: Hoboken, NJ, 2010, pp. 225-245.
- Danielson, N. D.; Gallagher, P. A.; Bao, J. J. Chemical Reagents and Derivatization Procedures in Drug Analysis. In Encylcopedia of Analytical Chemistry; Meyers, R. A., Ed.; John Wiley & Sons: Hoboken, NJ, 2000, pp. 7042-7076.
- Sellers, K. Why Derivatize? Improve GC Separations with Derivatization. Restek Corporation, 2010. www.restek.com/pdfs/CFTS1269.pdf (accessed May 23, 2017).
- Ding, J., et al. Current Progress on the Detection of Glyphosate in Environmental Samples. J. Science and Applications: BioMedicine, 2015, 3, 88-95. Available online at inter-use.com/Journals/JSAB/2015/Volume_03_Issue_06/2015/1001/103.html (accessed May 23, 2017).
- Benbrook, C. M. Trends in Glyphosate Herbicide Use in the United States and Globally. Environmental Sciences Europe 2016, 28(3). 15 pages. Available online at https://enveurope.springeropen.com/articles/10.1186/s12302-016-0070-0 (accessed May 23, 2017).
- https://commons.wikimedia.org/w/index.php?curid=9018141 (accessed May 23, 2017).
- TOXNET, Hazardous Compounds Data Bank, US National Library of Medicine (NIH). https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+1071-83-6 (accessed May 23, 2017).
- International Programme on Chemical Safety, Environmental Health Criteria 159: Glyphosate. http://www.inchem.org/documents/ehc/ehc/ehc159.htm#SectionNumber:1.1 (accessed May 23, 2017).
- Waiman, C. V, et al. A Simple and Rapid Spectrophotometric Method to Quantify the Herbicide Glyphosate in Aqueous Media: Application to Adsorption Isotherms on Soils and Goethite. Geoderma 2012, 170, 154-158.
- Restek Corporation, 2000. A Technical Guide for Headspace Analysis Using GC. www.restek.com/pdfs/59895B.pdf (accessed May 23, 2017).
- Sigma-Aldrich.com. Derivatization Reagents for Selective Response and Detection in Complex Matrices. http://www.sigmaaldrich.com/content/dam/sigma-aldrich/migrationresource4/Derivatization%20Rgts%20brochure.pdf (accessed May 23, 2017).
- Lin, D-L, et al. Chemical Derivatization for the Analysis of Drugs by GC-MS – A Conceptual Review. J. Food and Drug Analysis 2008, 16, 1-10. Available online at http://ir.fy.edu.tw/ir/retrieve/13771/8.pdf (accessed May 23, 2017).
- Orata, F. Derivatization Reactions and Reagents for Gas Chromatography Analysis. In Advanced Gas Chromatography – Progress in Agricultural, Biomedical, and Industrial Applications; Ali Mohd, M, Ed.; InTech Open.com, 2012. Available online at http://cdn.intechopen.com/pdfs/32817.pdf (accessed May 23, 2017).
- Regis Technologies, Inc. Chromatograph Catalog, GC Derivatization Reagents, pp. 44-58. http://www.registech.com/Magazine2/index.html#/0 (accessed May 23, 2017).
- Macherey-Nagel Gmbh & Co., MN Application Database. www.mn-net.com/apps/Searchapplications/tabid/10287/language/en-US/Default.aspx (accessed May 23, 2017).
- Christie, W. W. Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis. In Advances in Lipid Methodology – Two, 1993, pp. 69-111. Available online at aocs.files.cms-plus.com/LipidsLibrary/images/Importedfiles/lipidlibrary/topics/ester_93/file.pdf (accessed May 23, 2017).
- www.mpbio.com/product.php?pid=02157815&country=223 (accessed May 23, 2017).
- Plotka-Waslylka, J. M.; Morrison, C.; Biziuk, M; Namiesnik, J. Chemical Derivatization Processes Applied to Amine Determination in Samples of Different Matrix Composition. Chem. Rev. 2015, 115, 4693-4718.
- Coppex, L. Derivatives for HPLC Analysis. Ph.D. thesis; University of Geneva: Geneva, 1999.
- Toyo’oka, T., Editor. Modern Derivatization Methods for Separation Sciences, John Wiley & Sons: Chichester, West Sussex, England; 1999.
- Jalalizadeh, H., et al. A Stability-Indicating HPLC Method for the Determination of Memantine Hydrochloride in Dosage Forms through Derivatization with 1-Fluoro-2,4-dinitrobenzene. Sci. Pharm. 2014, 82, 265-279.
- Akhlaghi, Y., Ghaffari, S., Attar, H., Hoor, A. A. A rapid hydrolysis method and DABS-Cl derivatization for complete amino acid analysis of octreotide acetate by reversed phase HPLC. Amino Acids 2015, 47, 2255-2263.
- Durst, H. D., et al. Phenacyl Esters of Fatty Acids via Crown Ether Catalysts for Enhanced Ultraviolet Detection in Liquid Chromatography. Analytical Chemistry 1975, 47, 1797-1801.
- Huang, T., Yang, B., Yu, Y., Zheng, X, Duan, G. Reverse-phase high performance liquid chromatography for the determination of tiopronin in human plasma after derivatization with p-bromophenacyl bromide. Analytical Chimica Acta, 2006, 565, 178-182.
- Wong, J-M. T., et al. Benzoyl chloride derivatization with liquid chromatography-mass spectrometry for targeted metabolomics of neurochemicals in biological samples. J. Chromatography A, 2016, 1446, 78-90.
- Song, P., Mabrouk, O. S., Hershey, N. D., Kennedy, R. T. In Vivo Neurochemical Monitoring Using Benzoyl Chloride Derivatization and Liquid Chromatography-Mass Spectrometry. Analytical Chemistry, 2012, 84, 412-419.
- Koros, A., et al. Analysis of amino acids and biogenic amines in biological tissues as their o-phthalaldehyde/ethanethiol/fluorenylmethyl chloroformate derivatives by high-performance liquid chromatography: A deproteinization study. J. Chromatography A, 2007, 1149, 46-55.
- Uchiyama, S., Inaba, Y., Kunugita, N. Derivatization of carbonyl compounds with 2,4-dinitrophenylhydrazine and their subsequent determination by high-performance liquid chromatography. J. Chromatography B, 2011, 879, 1282-1289.
- Zhang, T., Jiang, G. Quantitative Analysis of Carbonyl-DNPH Derivatives by UHPLC/UV. Thermo Scientific Application Note 52007, 2010; available online at https://www.thermofisher.com.au/Uploads/file/Scientific/Applications/Scientific-Instruments-Automation/Quantitative-Analysis-of-Carbonyl-DNPH-Derivatives-by-UHPLC-UV.PDF (accessed June 8, 2017).
- Sirju, A.-P., Shepson, P. B. Laboratory and Field Investigation of the DNPH Cartridge Technique for the Measurement of Atmospheric Carbonyl Compounds. Environ. Sci. Technol. 1995, 29, 384-392.
- Determination of Carbonyl Compounds by High Performance Liquid Chromatography (HPLC). EPA Method 8315A, 1996; available online at https://www.epa.gov/sites/production/files/2015-07/documents/epa-8315a.pdf (accessed June 8, 2017).
- Schuster, R.; Schulenberg-Schell, H. Fluorescence Detection in Liquid Chromatography: A New Approach to Lower Limits of Detection and Easy Spectral Analysis, A Primer. Agilent Technologies, Publication Number 5968-9346E, 2000; available online at quimica.udea.edu.co/~carlopez/cromatohplc/fluorescence_primer.pdf (accessed August 1, 2018).
- Gautier, D.; Gupta, R. Determination of Gabapentin in Plasma by Liquid Chromatography with Fluorescence Detection after Solid-Phase Extraction with a C18 Column. Clinical Chemistry, 2002, 48, 2259-2261.
- Abdine, H.; Sultan, A.; Hefnawy, M. M.; Belal, F. Spectrophotometric determination of some β-blockers in tablets and human plasma using 9,10-dimethoxyanthracene-2-sodium sulfonate. Pharmazie, 2004, 60, 265-268.
- de Montigny, P.; Stobaugh, J. F.; Givens, R. S.; Carlson, R. G.; Srinivasachar, K.; Sternson, L. A.; Higuchi, T. Naphthalene-2,3-dicarboxyaldehyde/Cyanide Ion: A Rationally Designed Fluorogenic Reagent for Primary Amines. Anal. Chem., 1987, 59, 1096-1101.
- Li, J., et al.; Naphthalene-based fluorescent probes for glutathione and their applications in living cells and patients with sepsis. Theranostics, 2018, 8, 1411-1420.
- Cayman Chemical, 2015. Product Information, Monobromobimane. https://www.caymanchem.com/pdfs/17097.pdf (accessed August 1, 2018).
- Shen, X.; Pattillo, C. B.; Pardue, S.; Bir, S. C.; Wang, R.; Kevil, C. G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med, 2011, 50, 1021-1031.
- Almudever, P.; Peris, J-E.; Garrigues, T.; Diez, O.; Melero, A.; Alos, M. Quantification of nortriptyline in plasma by HPLC and fluorescence detection. J. Chromatogr. B, 2010, 878, 841-844.
- Kang, X.; Xiao, J.; Huang, X.; Gu, Z. Optimization of dansyl derivatization and chromatographic conditions in the determination of neuroactive amino acids of biological samples. Clinica Chimica Acta, 2006, 366, 352-356.
- Molins-Legua, C.; Campins-Falco, P.; Sevillano-Cabeza, A.; Pedron-Pons, M. Urine polyamines determination using dansyl chloride derivatization in solid-phase extraction cartridges and HPLC. Analyst, 1999, 124, 477-482.
- PerkinElmer, Inc. Fluorescence Derivatisation in Liquid Chromatography. 2000; available online at quimica.udea.edu.co/~carlopez/cromatohplc/hplc_deri_fluorescen.pdf (accessed August 1, 2018).
- Huang, C-C. Polyneuropathy Induced by n-Hexane Intoxication in Taiwan. Acta Neurologica Taiwanica, 2008, 17, 3-10.
- Harris, O. M.; Corcoran, J. Toxicological Profile for n-Hexane. U. S. Department of Health and Human Services, 1999; available online at http://www.atsdr.cdc.gov/toxprofiles/tp113.pdf (accessed August 1, 2018).
- Maestri, L.; Imbriani, M.; Capodaglio, E. Determination of 2,5-hexanedione by high-performance liquid chromatography after derivatization with dansylhydrazine. J. Chromatogr B Biomed Appl, 1994, 657, 111-117.
- Rose, M. J.; Lunte, S. M.; Carlson, R. G.; Stobaugh, J. F. Hydroquinone-Based Derivatization Reagents for the Quantitation of Amines Using Electrochemical Detection. Anal. Chem. 1999, 71, 2221-2230.
- Anderson, G. M.; Young, J. G. Determination of Neurochemically Important Compounds in Physiological Samples Using HPLC. Schizophrenia Bulletin 1982, 8, 333-348. Available online at https://academic.oup.com/schizophreniabulletin/article/8/2/333/1911946 (accessed July 9, 2018).
- Keller, R.; Oke, A; Mefford, I.; Adams, R. N. Liquid Chromatographic Analysis of Catecholamines: Routine Assay for Regional Brain Mapping. Life Sciences, 1976, 19, 995-1004.
- Kissinger, P. T.; Bruntlett, C. S.; Shoup, R. E. Minireview: Neurochemical Applications of Liquid Chromatography with Amperometric Detection. Life Sciences, 1981, 28, 455-465.
- Acworth, I. N.; Waraska, J. Electrochemical Measurement of Electrochemically Unreactive Compounds: An Examination of the Use of Derivatives and Photolysis with HPLC and Coulometric Detection. In Progress in HPLC-HPCE, Vol. 6; Acworth, I. N. et al. (Eds); VSP BV: Zeist, The Netherlands, 1997, pp. 351-376.
- Krull, I. S.; Selavka, C. M.; Duda, C.; Jacobs, W. Derivatization and Post-Column Reactions for Improved Detection in Liquid Chromatography/Electrochemistry. J. Liquid Chromatography, 1985, 8, 2845-2870.
- Seiwert, B.; Karst, U. Ferrocene-Based Derivatization in Analytical Chemistry. Anal. Bioanal. Chem., 2008, 390, 181-200.
- Fuchigami, T.; Atobe, M.; Inagi, S. Fundamentals and Applications of Organic Electrochemistry: Synthesis, Materials, Devices, John Wiley and Sons: 2015, Appendix B, Tables of Physical Data, p.217. Available online at https://onlinelibrary.wiley.com/doi/pdf/10.1002/9781118670750.app2 (accessed September 13, 2018).
- Sierra, T.; Crevillen, A. G.; Escarpa, A. Derivatization Agents for Electrochemical Detection in Amino Acid, Peptide, and Protein Separations: The Hidden Electrochemisty. Electrophoresis, 2017, 0, 1-9.
- Poole, C.F. Derivatization in Liquid Chromatography. In Liquid Chromatography: Applications, Fanali, S., et al., Eds.; Elsevier, Waltham, MA, 2013, pp. 25-56.
- Oppolzer, D. J. Detection of Biogenic Amines in Urine and Plasma by Liquid Chromatography Coupled to Electrochemical Detection (HPLC-ED) Using Microextraction in Packed Syringe (MEPS). Masters thesis; Universidade da Beira Interior: Covilhã, Portugal, 2012. Available online at https://ubibliorum.ubi.pt/bitstream/10400.6/2870/1/Tese%20final%20david.pdf (accessed July 9, 2018).
- Amuza Neuroscience, 2018. HPLC-EC (Electrochemical Detection) Fundamentals. https://www.eicomusa.com/hplc-ecd/electrochemical-detection-ecd-fundamentals/ (accessed July 9, 2018).
- Honeychurch, K. Review: The Application of Liquid Chromatography Electrochemical Detection for the Determination of Drugs of Abuse. Separations, 2016, 3, 28; doi:10.3390/separations 3040028. Available online at http://www.mdpi.com/2297-8739/3/4/28 (accessed July 11, 2018).
- Flanagan, R. J.; Perrett, D.; Whelpton, R. Electrochemical Detection in HPLC: Analysis of Drugs and Poisons, Royal Society of Chemistry: Cambridge, 2005, pp. 56-78.
- Chen, R.; Deng, Y.; Yang, L.; Xu, F. Determination of Histamine by High-Performance Liquid Chromatography After Precolumn Derivatization with o-Phthalaldehyde-Sulfite. J. Chromatographic Science, 2016, 54, 547-553.
- Shimada, K.; Matsue, T.; Shimada, K. Reagents for Electrochemical Detection. In Modern Derivatization Methods for Separation Sciences, Toyo’oka, T., Ed.; John Wiley & Sons: West Sussex, England, 1999, pp. 191-216.
- Zanello, P., et al. The Redox Behaviour of Ferrocene Derivatives, VI: Benzylferrocenes. The Crystal Structure of Decabenzylferrocenium Tetrafluoroborate. J. Organometallic Chem., 1994, 171-177.
- van Staveren, D. R.; Netzler-Nolte, N. Bioorganometallic Chemistry of Ferrocene. Chem. Rev., 2004, 104, 5931-5835.
- Metzler-Nolte, N. Conjugates of Peptides and PNA with Organometallic Complexes: Synthesis and Applications. In Bioorganometallics: Biomolecules, Labeling, Medicine, Jaouen, G., Ed. Wiley-VCH, Weinheim, 2006.
- Munns, R. K.; Roybal, J. E.; Shimoda, W.; Hurlbut, J. A. 1-(4-Hydroxyphenyl)-1-(2,4-dihydroxyphenyl)- and 1-(2,5-dihydroxyphenl)-2-bromoethanones: New Labels for Determination of Carboxylic Acids by High-performance Liquid Chromatography with Electrochemical and Ultraviolet Detection. J. Chromatography, 1988, 442, 209-218.
- Shimada, K.; Tanaka, M.; Nambara, T. Studies on Steroids. CC. Determination of 17-Ketosteroid Sulphates in Serum by High-performance Liquid Chromatography with Electrochemical Detection Using Pre-column Derivatization. J. Chromatography, 1984, 307, 23-28.
- Lunn, G.; Hellwig, L. C., Eds. Handbook of Derivatization Reactions for HPLC. John Wiley & Sons, New York, 1998.


