6.2: Separation and confirmation of individual ions in group IV precipitates and group V mixture
- Page ID
- 369549
Separating and confirming barium ion
The precipitates of Group IV cations, i.e., \(\ce{CaCO3}\) and \(\ce{BaCO3}\) are soluble in acidic medium. In these experiments acetic acid \(\ce{CH3COOH}\) is used to make the solution acidic that results in the dissolution of \(\ce{CaCO3}\) and \(\ce{BaCO3}\):
\[\ce{4CH3COOH(aq) + 4H2O(l) <=> 4CH3COO^{-}(aq) + 4H3O^{+}(aq)}\nonumber\]
\[\ce{CaCO3(s, white) <=> Ca^{2+}(aq) + CO3^{2-}(aq)}\nonumber\]
\[\ce{BaCO3(s, white) <=> Ba^{2+}(aq) + CO3^{2-}(aq)}\nonumber\]
\[\ce{2CO3^{2-}(aq) + 4H3O^{+}(aq) <=> 2H2CO3(aq) +4H2O(l)}\nonumber\]
\[\ce{2H2CO3(aq) <=> 2H2O(l) + 2CO2(g)(^)}\nonumber\]
\[\text{Overall reaction: }\ce{~4CH3COOH(aq) + CaCO3(s, white) + BaCO3(s, white) <=> 4CH3COO^{-}(aq) + Ca^{2+} + Ba^{2+} + 2H2O(l) + 4CO2(g)(^)}\nonumber\]
\(\ce{CO3^{2-}}\) ion is a weak base that reacts with \(\ce{H3O^{+}}\) and forms carbonic acid (\(\ce{H2CO3}\). Carbonic acid is unstable in water and decomposes into carbon dioxide and water. Carbon dioxide leaves the solution that drives the reactions forward, as shown in Figure \(\PageIndex{1}\).

The Acetate ion (\(\ce{CH3COO^{-}}\)) produced in the above reactions is a conjugate base of a weak acid acetic acid (\(\ce{CH3COOH}\)). More acetic acid is added to the solution to make a \(\ce{CH3COOH}\)/\(\ce{CH3COO^{-}}\) buffer that can maintain \(pH\) at ~5.
Potassium chromate (\(\ce{K2CrO4}\)) solution is added at this stage that introduces chromate ion \(\ce{CrO4^{2-}}\):
\[\ce{K2CrO4(s) <=> 2K^{+}(aq) + CrO4^{2-}(aq)}\nonumber\]
Although both calcium and barium ions form insoluble salt with chromate ion (\(\ce{CaCrO4}\) \(K_{sp}\) = 7.1 x 10-4 and \(\ce{BaCrO4}\) \(K_{sp}\) = 1.8 x 10-10), \(\ce{BaCrO4}\) is less soluble and can be selectively precipitated by controlling \(\ce{CrO4^{2-}}\) concentration. The chromate ion is involved in the following \(pH\) dependent equilibrium:
\[\ce{2CrO4^{2-}(aq) + 2H3O^{+}(aq) <=> Cr2O7^{2-}(aq) + 3H2O(l)}\quad K = 4.0\times10^{14}\nonumber\]
At \(pH\) ~5 in a \(\ce{CH3COOH}\)/\(\ce{CH3COO^{-}}\) buffer, the concentration of \(\ce{CrO4^{2-}}\) is enough to selectively precipitate barium ions leaving calcium ions in the solution:
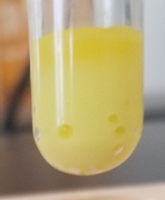
\[\ce{Ba^{2+}(aq) + CrO4^{2-}(aq) <=> BaCrO4(s, light~yellow)(v)}\nonumber\]
The mixture is centrifuged and decanted to separate \(\ce{BaCrO4}\) precipitate from the supernatant containing \(\ce{Ca^{2+}}\) ions as shown in Figure \(\PageIndex{2}\). Although the formation of a light yellow precipitate (\(\ce{BaCrO4}\) ) at this stage is a strong indication that \(\ce{Ba^{2+}}\) is present in the test sample, \(\ce{Ca^{2+}}\) may also form a light yellow precipitate (\(\ce{CaCrO4}\) ), particularly if pH is higher than the recommended value of 5.

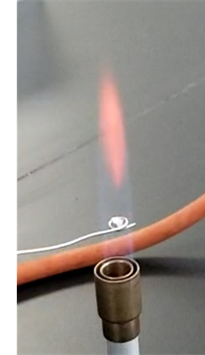
Group IV and V cations are most often confirmed by the flame test. Figure \(\PageIndex{3}\) shows the flame test results of group IV cations. The presence of barium is further confirmed by a flame test. For this purpose, \(\ce{BaCrO4}\) precipitate is treated with 12M \(\ce{HCl}\). The concentrated \(\ce{HCl}\) removes \(\ce{CrO4^{2-}}\) by converting it to dichromate (\(\ce{Cr2O7^{2-}}\)) that resulting in the dissolution of \(\ce{BaCrO4}\):
\[\ce{2BaCrO4(s, light~yellow) <=> 2Ba^{2+}(aq) + 2CrO4^{2-}(aq)}\nonumber\]
\[\ce{2CrO4^{2-}(aq) + 2H3O^{+}(aq) <=> Cr2O7^{2-}(aq) + 3H2O(l)}\nonumber\]
A flame test is applied to the solution. \(\ce{Ba^{2+}}\) imparts characteristic yellow-green color to the flame. If the yellow-green color is observed in the flame test, it confirms \(\ce{Ba^{2+}}\) is present in the test sample.


Confirming calcium ion
The \(\ce{Ca^{2+}}\) present in the supernatant is precipitated by adding oxalate ion (\(\ce{C2O4^{2-}}\)):
\[\ce{Ca^{2+}(aq) + C2O4^{2-}(aq) <=> CaC2O4(s, white)(v)}\nonumber\]


The formation of white precipitate, i.e., \(\ce{CaC2O4}\) shown in Figure \(\PageIndex{4}\), is a strong indication that \(\ce{Ca^{2+}}\) is present in the test sample. However, if \(\ce{Ba^{2+}}\) is not fully separated earlier, it also forms a white precipitate \(\ce{BaC2O4}\). The presence of \(\ce{Ca^{2+}}\) is further verified by flame test. For this purpose, the precipitate is dissolved in 6M \(\ce{HCl}\):
\[\ce{CaC2O4(s, white) <=> Ca^{2+}(aq) + C2O4^{2-}(aq)}\nonumber\]
\[\ce{C2O4^{2-}(aq) + 2H3O^{+}(aq) <=> H2C2O4(aq)}\nonumber\]
Strong acid like \(\ce{HCl}\) increases \(\ce{H3O^{+}}\) ion concentration that drives the above reaction forward based on Le Chatelier's principle. The flame test is applied to the solution. If \(\ce{Ca^{2+}}\) is present in the solution, it imparts characteristic brick-red color to the flame, as shown in Fig. 6.2.3. Observation of the brick-red color in the flame test confirms the presence of \(\ce{Ca^{2+}}\) in the test sample. The flame color changes to light green when seen through cobalt blue glass.
Confirming group V cations by the flame test
Group V cations, i.e., alkali metal, \(\ce{Na^{+}}\), \(\ce{K^{+}}\), etc. form soluble ionic compounds. Separation of alkali metals cations by selective precipitation is not possible using commonly available reagents. So, group V cations are not separated in these analyses. However, alkali metal cations impart characteristic color to the flame that helps in their confirmation as shown in Figure \(\PageIndex{5}\). The supernatant after separating group IV precipitate is concentrated by heating the solution to evaporate the solvent. A flame test is applied to the concentrated solution.
Lithium imparts carmine red, sodium imparts intense yellow, and potassium imparts lilac color to the flame.




