1.2: X-rays
- Page ID
- 352421

An unexpected result! Discovery of X-rays in 1895.
(Illustration by Alejandro Martínez de Andrés, CSIC 2014)
By the end of the 19th century, in 1895, Wilhelm Conrad Röntgen (1845-1923), a German scientist from the University of Würzburg, discovered a form of radiation (of unknown nature at that time, and hence the name X-rays) which had the property of penetrating opaque bodies. In the first paragraph of his communication sent to the Society of Physics and Medicine of Wurzburg (1895) he reports the discovery as follows:
After producing an electrical discharge with a Ruhmkorff’s coil through a Hittorf’s vacuum tube, or a sufficiently evacuated Lenard, Crookes or similar apparatus, covered with a fairly tight-fitting jacket made of thin, black paperboard, one sees that a cardboard sheet coated with a layer of platinum and barium cyanide, located in the vicinity of the apparatus, lights up brightly in the completely darkened room regardless of whether the coated side is pointing or not to the tube. This fluorescence occurs up to 2 meters away from the apparatus. One can easily be convinced that the cause of the fluorescence proceeds from the discharge apparatus and not from any other source of the line.
To learn about some aspects of the discovery, as well as about personal aspects of Röntgen, see also the chapter dedicated to some biographical outlines. But if you can read Spanish, there is an extensive chapter dedicated to both the historical details around Röntgen and his discovery.
- Left: Wilhelm Conrad Röntgen (1845-1923), around 1895 with an X-ray photograph of his wife's hand showing her wedding ring . For his discovery Röntgen won the Nobel Prize in Physics in 1901.
- Right: Typical hospital radiology equipment
X-rays are invisible to our eyes but they can produce visible images if we use photographic plates or special detectors...
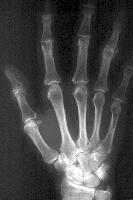
Left: Radiographic image of a hand
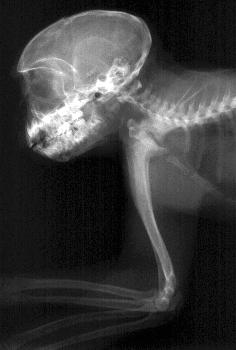
Right: Radiographic image of a monkey


Left: Radiographic image of a well-done weld
Right: Poorly-done weld (black line)
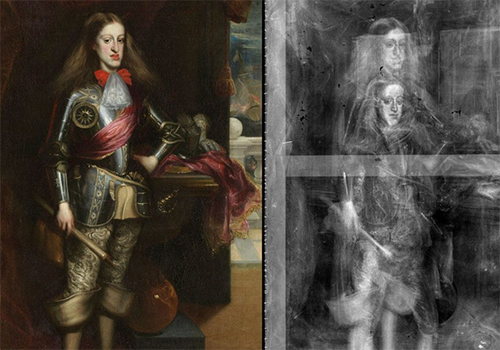
A painting and its X-ray photograph showing two superimposed paintings on the same canvas
(Charles II of Spain, by Carreño de Miranda, Museo del Prado, Madrid)
We all know several applications of X-rays in the medical field: angiography (the study of blood vessels) or the so-called CT scans, but the use of X-rays has also been extended to detect failures in metals or for the analysis of paintings.
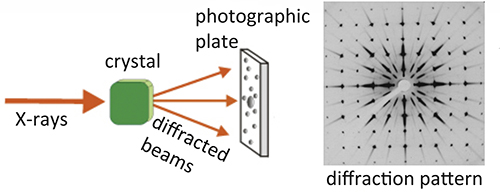
Many years passed from the discovery of X-rays in 1895 until that finding produced a revolution in the fields of Physics, Chemistry and Biology. The potential applications in these areas came in 1912 indirectly from the hand of Max von Laue (1879-1960), professor at the Universities of Munich, Zurich, Frankfurt, Würzburg and finally Berlin.
Paul Peter Ewald (1888-1985) got his friend, Max Laue, interested in his own experiments on the interference between radiations with large wavelengths (practically visible light) on a "crystalline" model based on resonators (note that at that time the question on wave-particle duality was also under discussion). The idea then came to Laue that the much shorter electromagnetic rays, which X-rays were supposed to be, would cause some kind of diffraction or interference phenomena in a medium, and that a crystal could provide this medium.
Left: Max von Laue (1879-1960)
Right: Paul. P. Ewald (1888-1985)
Max von Laue demonstrated the nature of this new radiation by putting crystals of copper sulfate, and of the mineral zinc blende, in front of an X-ray source, obtaining confirmation of his hypothesis and demonstrating both, the undulatory nature of this radiation and the periodic nature of crystals. For these findings he received the Nobel Prize in Physics in 1914.
Left: William H. Bragg (1862-1942)
Right: William L. Bragg (1890-1971)
However, those who really benefited from the discovery of the Germans were the British Braggs (father and son), William H. Bragg (1862-1942) and William L. Bragg (1890-1971), who together in 1915 received the Nobel Prize in Physics for demonstrating the usefulness of the phenomenon discovered by von Laue for obtaining the internal structure of crystals - but all this will be the subject of later chapters.
This chapter will deal exclusively with the nature and production of X-rays...
X-rays are electromagnetic radiations, of the same nature as visible light, ultraviolet or infrared radiations, and the only thing that distinguishes them from other electromagnetic radiations is their wavelength, which is about 10-10 m (equivalent to the unit of length known as one Angstrom).

Graphic representation of an electromagnetic wave, showing its associated electric (E) and magnetic (H) fields, moving forwards at the speed of light.

The continuous spectrum of visible light (wavelength decreases from red to violet )
Excellent information on the electromagnetic spectrum can be found in some pages offered by NASA. The reader can also learn about X-rays and their applications in Medical Radiography and in the pages of The X-Ray Century.
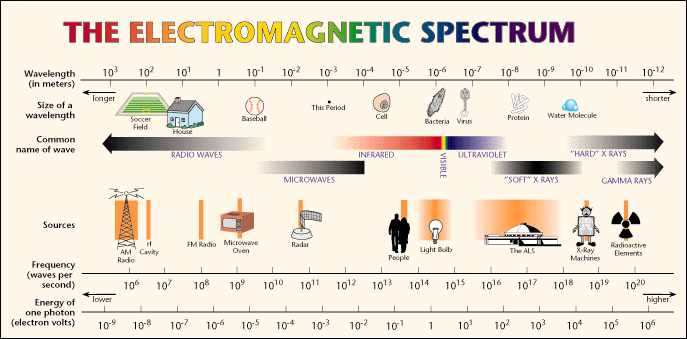
ν(Hz) λ(m) = 3 108 m Hz
E(J) = h(J/Hz) ν(Hz) = k(J/K molecule) T(K)
h = 6.6 10-34 (J/Hz); k = 1.4 10-23 (J/K molecule); 1 eV = 1.6 10-19 (J)
Figure taken from the Berkeley Lab
The most interesting X-rays for Crystallography are those having a wavelength close to 1 Angstrom (the hard X-rays in the diagram above), which is a distance very close to the interatomic distances occurring in molecules and crystals. These type of X-rays have a frequency of approximately 3 million THz (tera-hertz) and to an energy of 12.4 keV (kilo-electron-volts), which in turn would correspond to a temperature of about 144 million degrees Celsius. These wavelengths are produced in Crystallography laboratories and in large synchrotrons as ESRF, ALBA, Diamond, DESY, ...

X-ray generator in a Crystallography laboratory. The goniometric and detection systems are shown behind the X-ray tube.

Aerial photograph of the synchrotron at the ESRF in Grenoble (France). Note its circular geometry


Conventional X-ray tubes used for crystallographic studies during the 20th century
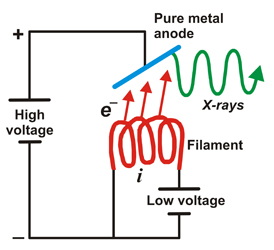
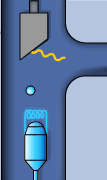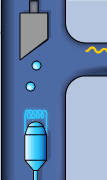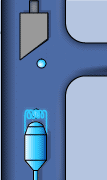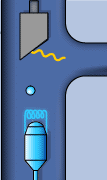
Static sketch and animation of the X-ray production on a conventional X-ray tube
Those 50 kV are supplied as a potential difference (high voltage) between an incandescent filament (through which a low voltage electrical current of intensity i passes: around 5 A at 12 V) and a pure metal (usually copper or molybdenum). This produces an electrical current (of free electrons) between them of about 30 mA. From the incandescent filament (negatively charged) the free electrons jump to the anode (positively charged) causing (in the pure metal) a reorganization in its electronic energy levels.
This is a process that generates a lot of heat, so that X-ray tubes must be very well chilled. An alternative to conventional X-ray tubes are the rotating anode generators, in which the anode in the form of a cylinder is maintained in a continuous rotation, so that the incidence of electrons is distributed over its cylindrical surface and thus a higher power can be obtained.
Left: Rotating anode generator
Right: Rotating anode of polished copper (images taken from Bruker-AXS)
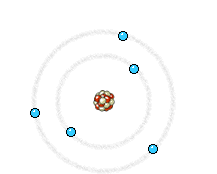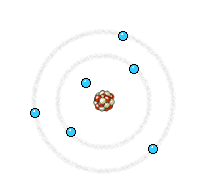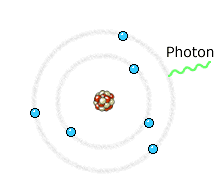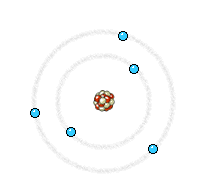
The so-called "characteristic X-rays" are produced according to the following scheme:

a) Energy state of electrons in an atom of the anode that is going to be reached by an electron from the filament. b) Energy state of the same electrons after impact with the electron from the filament. The incident electron bounces and ejects an electron from the anode, producing the corresponding hole. c) An electron of a higher energy level falls and occupies the hole. This energy jump, perfectly defined, generates the so-called characteristic X-rays of the anodic material.
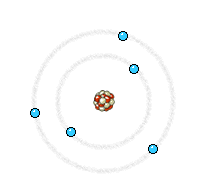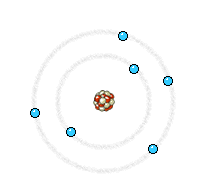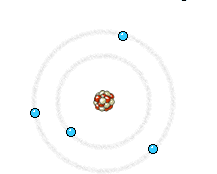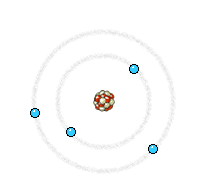
Left: In an X-ray tube the electrons emitted from the cathode are accelerated towards the metal target anode by an accelerating voltage of typically 50 kV. The high energy electrons interact with the atoms in the metal target. Sometimes the electron comes very close to a nucleus in the target and is deviated by the electromagnetic interaction. In this process, which is called bremsstrahlung (braking radiation), the electron loses much energy and a photon (X-ray) is emitted. The energy of the emitted photon can take any value up to a maximum corresponding to the energy of the incident electron.
Right: The high energy electron can also cause an electron close to the nucleus in a metal atom to be displaced. This vacancy is filled by an electron further out from the nucleus. The well defined difference in binding energy, characteristic of the material, is emitted as a monoenergetic photon. When detected this X-ray photon gives rise to a characteristic X-ray line in the energy spectrum. Animations taken from Nobelprize.org.
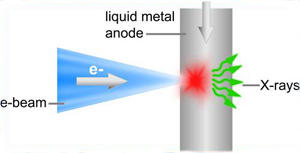
Apart from the developments made on the new synchrotron sources, there still exist several attempts to optimize efficiency and power of the "in-house" X-ray sources, as the ones based on the microfocus technology, that is, high brightness sources that additionally use very stable optics mounted to the tube housing, or those based on the use of a liquid metal as anode...
Left: New microfocus X-ray tube. Image taken from Incoatec
Right: New development for an of X-ray source based on liquid metal anodes.
Taken from Excillum. There is an animation showing this technology
The energetic restoration of the excited anodic electron is carried out with an X-ray emission with a frequency that corresponds exactly to the specific energy gap (quantum) that the electron needs to return to its initial state. These X-rays therefore show a specific wavelength and are known as characteristic wavelengths of the anode. The most important characteristic wavelengths in X-ray Crystallography are the so-called K-alpha lines (Kα), produced by the electrons falling to the innermost layer of the atom (higher binding energy). However, in addition to these specific wavelengths, a continuous range of wavelengths, very close to each other, is also produced known as the continuous radiation which is due to the braking of the incident electrons when they hit the metal target.
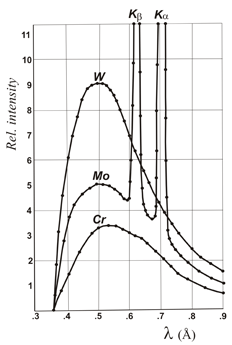
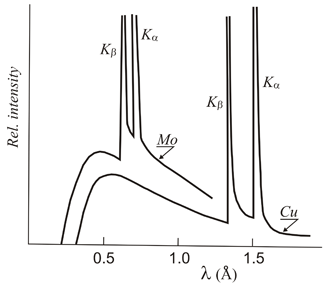
Distribution of X-ray wavelengths produced in a conventional X-ray tube where the anode material is copper (Cu), molybdenum (Mo), chromium (Cr) or tungsten (W). Over the so-called continuous spectrum, the characteristic K-alpha (Kα) and K-beta (Kβ) lines are shown. The starting point of the continuous spectrum appears at a wavelength which is approximately 12.4 / V, (Angstrom) where V represents the amount of kV between anode and filament. For a given voltage between the anode and filament, only the characteristic wavelengths of molybdenum are obtained (figure on the left).
In synchrotrons, the generation of X-rays is quite different. A synchrotron facility contains a large ring (on the order of kilometers), where electrons move at a very high speed in straight channels that occasionally break to match the curvature of the ring. These electrons are made to change direction to go from one channel to another using magnetic fields of high energy. It is at this moment, when electrons change their direction, that the electrons emit a very high energy radiation known as synchrotron radiation. This radiation is composed of a continuum of wavelengths ranging from microwaves to the so-called hard X-rays.
Synchrotrons appearance is very similar to that shown in the following schemes:

A synchrotron scheme. The linear accelerator (Linac) and the circular accelerator (Booster) are seen in the center, surrounded by the outer storage ring. The emitted X-rays are directed to the beamlines.
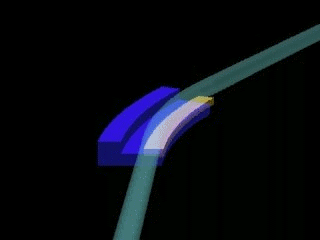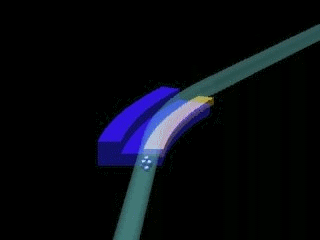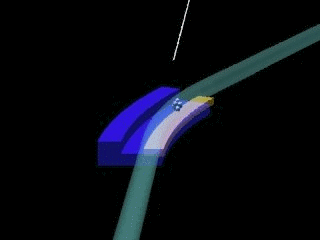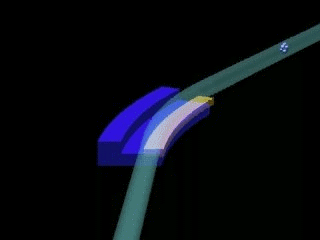
Left: General sketch of a synchrotron. The central circle is where the charged particles are accelerated (linac & booster). The outer circle is the storage ring, formed by crooked lines, at the end of which the experimental stations are installed.
Right: Outline of the junction of two crooked lines of the storage ring of a synchrotron. X-rays appear due to the change of direction of the charged particles.
The interested reader can access a demonstration on the operation of a synchrotron ring through this link, or see the same animation in a larger size through this other link.
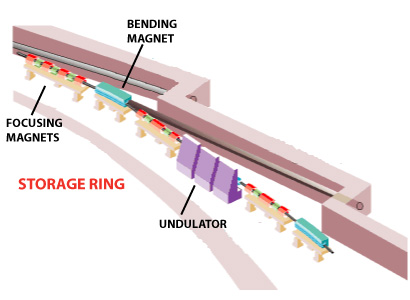
Outline of the point between two straight segments in the storage ring of a synchrotron. Image taken from the ESRF
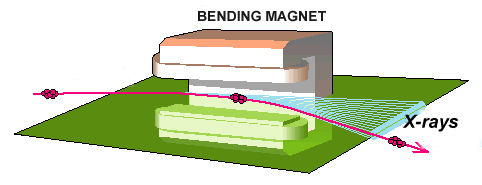
Details of how X-rays are produced in a synchrotron in the curvature of the electrons' trajectory inside the storage ring. Image taken from the ESRF
The X-rays obtained in the synchrotrons have two clear advantages for crystallography:
- the wavelengths can be tuned at will, and
- its brilliance is at least 1021 times higher that those obtained with a conventional X-ray tube (see the image below).
Here can you find a list of synchrotrons and storage rings used as synchrotron radiation sources, and free electron lasers around the world.

The brilliance of X-ray sources: conventional X-ray tubes, synchrotrons and the future XFEL. Image taken from the ESRF.
The following image shows an outline of an experimental station of a synchrotron: a) the optics hutch, where X-rays are filtered and focused using curved mirrors and monochromators; b) the experimental hutch, where the goniometer, sample and detector are located and where the diffraction experiment is done and, c) the control cabin, where the experiment is monitored and, if required, also evaluated.
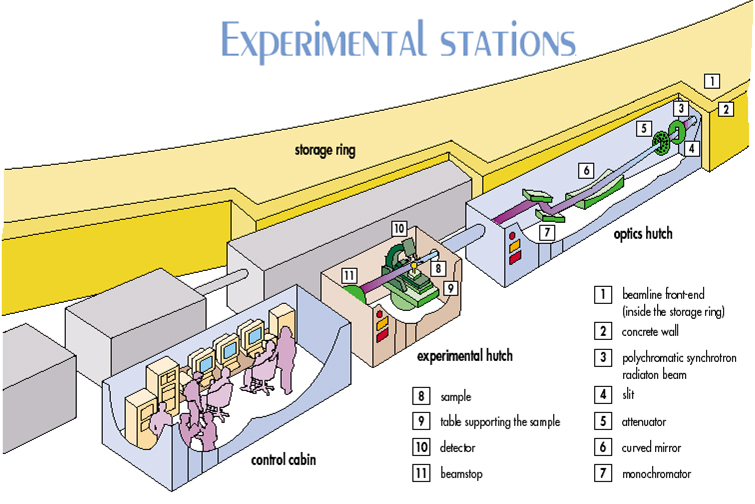
Outline of an experimental station in a synchrotron
Lightsources.org contains news and science highlights from each light source facility, as well as photos and videos, education and outreach resources, a calendar of conferences and events, and information on funding opportunities.
The radiation used for crystallography is usually monochromatic (or nearly monochromatic), that is, a radiation with exclusively (or almost exclusively) a single wavelength. In order to achieve this, the so-called monochromators are used, which consist of a system of crystals that, based on Bragg's Law (which will be presented in another chapter), are able to "filter" (through the interaction between the crystals and the X-rays) the polychromatic radiation, allowing only one wavelength (color), as shown below.

Outline of a monochromator. Polychromatic radiation (white) coming from the left (below) is "reflected" , in accordance with Bragg's Law, (to be seen in subsequent chapter), in different orientations of the crystal to produce ("to filter") a monochromatic radiation that is reflected again ("filtered") in the secondary crystal. For the moment it is enough that the reader is aware that this law will allow us to understand how the crystals "reflect" the X-rays, behaving as special mirrors . Image taken from the ESRF.
X-rays interact with the electrons of matter... A monochromatic beam (ie with a single wavelength) suffers an exceptional attenuation, proportional to the thickness being crossed. This attenuation may arise from several factors: a) the body heats up, b) a fluorescent radiation, with different wavelength, is produced & accompanied by photoelectrons, both being characteristic of the material (this leads to the photo-electron spectroscopies, Auger and PES); and c) scattered X-rays with the same wavelength (coherent and Bragg) or with slightly higher wavelengths (Compton), together with the scattered electrons.
Of all these effects, the most important one is fluorescence, where the absorption increases by increasing incident wavelength. However, this behavior has discontinuities (anomalous dispersion) for those energies that correspond to electronic transitions between different energy levels of the material (this leads to the EXAFS spectroscopy).
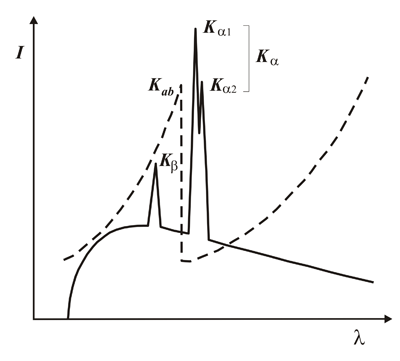
Spectrum emitted by a metallic anode showing its characteristic wavelengths (continuous line). In the same figure, but referred to a vertical axis of absorbance (not drawn) the increasing and discontinuous variation of the absorption (dashed line) of a given material is also shown. This gives an idea of the use of this property as a filter to obtain monochromatic radiation, at least separating the double Kα1 - Kα2 from the rest of the spectrum. This approach, using concrete materials with specific absorption capacities, was used in Crystallography laboratories until the early 1970's to obtain monochromatic radiation.
Special mention deserves the recent discovery introduced in the field of femtosecond X-ray protein nanocrystallography. Using this technique (XFEL: X-ray Free Electron Laser), based on the use of X-rays obtained from a free electron laser, "snapshots" of X-ray diffraction can be obtained in the femtoseconds scale. It has been proposed that femtosecond X-ray pulses can be used to outrun even the fastest damage processes by using single pulses so brief that they terminate before the manifestation of damage to the sample in less time than it needed to be damaged by the crystallites radiation.This will imply a giant step to remove virtually all the difficulties in the crystallization process, especially for proteins (see these articles: Nature (2011) 470, 73-77, Nature (2013) and Nature(2014)). In this sense, it is also worth quoting the article published in Radiation Physics and Chemistry (2004) 71, 905-916, which already warned on the future importance of the free electron laser on structural biology.
The European XFEL generates ultrashort X-ray flashes, 27,000 times per second and with a brilliance that is a billion times higher than that of the best conventional X-ray radiation sources. Thanks to its outstanding characteristics, which are unique worldwide, the facility opens up completely new research opportunities for scientists and industrial users. It could be interesting to look at the video offered on the web site of the international consortium, or directly through this link.
Regarding the use of these powerful X-ray sources for determining the structure of biological macromolecules, the interested readers should consider the very promising results published in Nature (2016) 530, 202-206. This study provides the opportunity to use not only the information contained in the diffraction spots generated by crystals, but also in the very weak intensity distribution found around and between the diffraction spots, the so called continuous diffraction.
With X-rays from free-electron lasers crystallographic applications are extended to nanocrystals, and even to single non-crystalline biological objects and even movies of biomolecules in action can be produced.
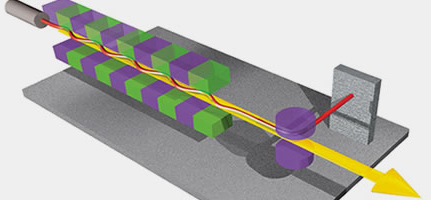
To generate the X-ray flashes, bunches of electrons will first be accelerated to high energies and then directed through special arrangements of magnets (undulators). In the process, the particles will emit radiation that is increasingly amplified until an extremely short and intense X-ray flash is finally created.
Recently, the modification that involves replacing the so-called material undulators (magnets) with a new optical device also based on laser technology, dramatically reduces the size of the XFEL by about 10,000 times and the size of the accelerator by 100 times, leading to an incredible reduction in size and price of the so called CXFEL (compact X-ray free-electron laser).
In any case, X-rays, like any light "illuminate" and "let to see", but in a different manner than we see with our eyes. We encourage you to go forward, to understand how X-rays allow us "to see" inside crystals, that is, to "see" the atoms and the molecules.