8: Main group oxidation states
- Page ID
- 149473
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\(\newcommand{\ket}[1]{\left| #1 \right>} \)
\( \newcommand{\bra}[1]{\left< #1 \right|} \)
\( \newcommand{\braket}[2]{\left< #1 \vphantom{#2} \right| \left. #2 \vphantom{#1} \right>} \)
\( \newcommand{\qmvec}[1]{\mathbf{\vec{#1}}} \)
\( \newcommand{\op}[1]{\hat{\mathbf{#1}}}\)
\( \newcommand{\expect}[1]{\langle #1 \rangle}\)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Main Group Oxidation States
The formal oxidation state is the hypothetical charge that an atom would have if all
bonds to atoms of different elements were 100% ionic. Some simple rules:
- For shared electrons, assign ALL electrons in the bond to the more electronegative atom and then do a formal charge calculation.
- If two identical atoms are bonded, split those electrons 50/50.
- Do a formal charge calculation.
- Now calculate the oxidation state for all atoms in the following species:

Oxidation States in Reactions
- For each of the following reactions:
- Determine the oxidation state of the atoms in the starting material.
- Determine the oxidation state of the atoms in the products.
- Determine which compound was oxidized/reduced/no change.
- Remember, in coordination compound, the ligand owns the donated pair.
- H2O + CO2 –> H2CO3
- CO2+ H2O -> C6H12O6+O2
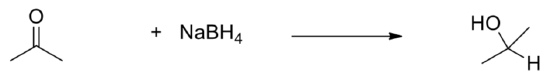
Oxidation Levels in Biological Cofactors
In more complicated molecules, it is easier to assess an overall oxidation level rather than thinking about the oxidation state of each atom.
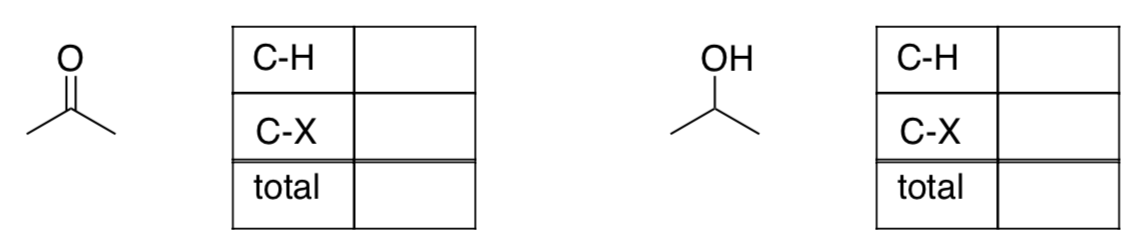
Each C-H bond can be thought of as C- H+. A bond to H means a negative charge on carbon.
EachC-Xbond(X=O,N,Cl,...)canbethoughtofasC+X-. AbondtoXmeansa positive charge on carbon.
Methane, CH4, is a common form of carbon. To assess its oxidation level, we would assign a -1 charge to the carbon for every C-H bond.
- Draw methane.
- Assess the oxidation level in methane.
C-H Add up the number of C-H bonds and multiply by -1 C-X Add up the C-X bonds Total
Carbon dioxide, CO2, is another common form of carbon. To assess its oxidation level, we would assign a +1 charge to the carbon for every C-O bond.
- Draw carbon dioxide
- Assess the oxidation level in carbon dioxide.
C-H Add up the number of C-H bonds and multiply by -1 C-X Add up the C-X bonds Total - More practice: What are the overall oxidation levels in acetone and isopropanol?
- Practice with the following biological cofactors.
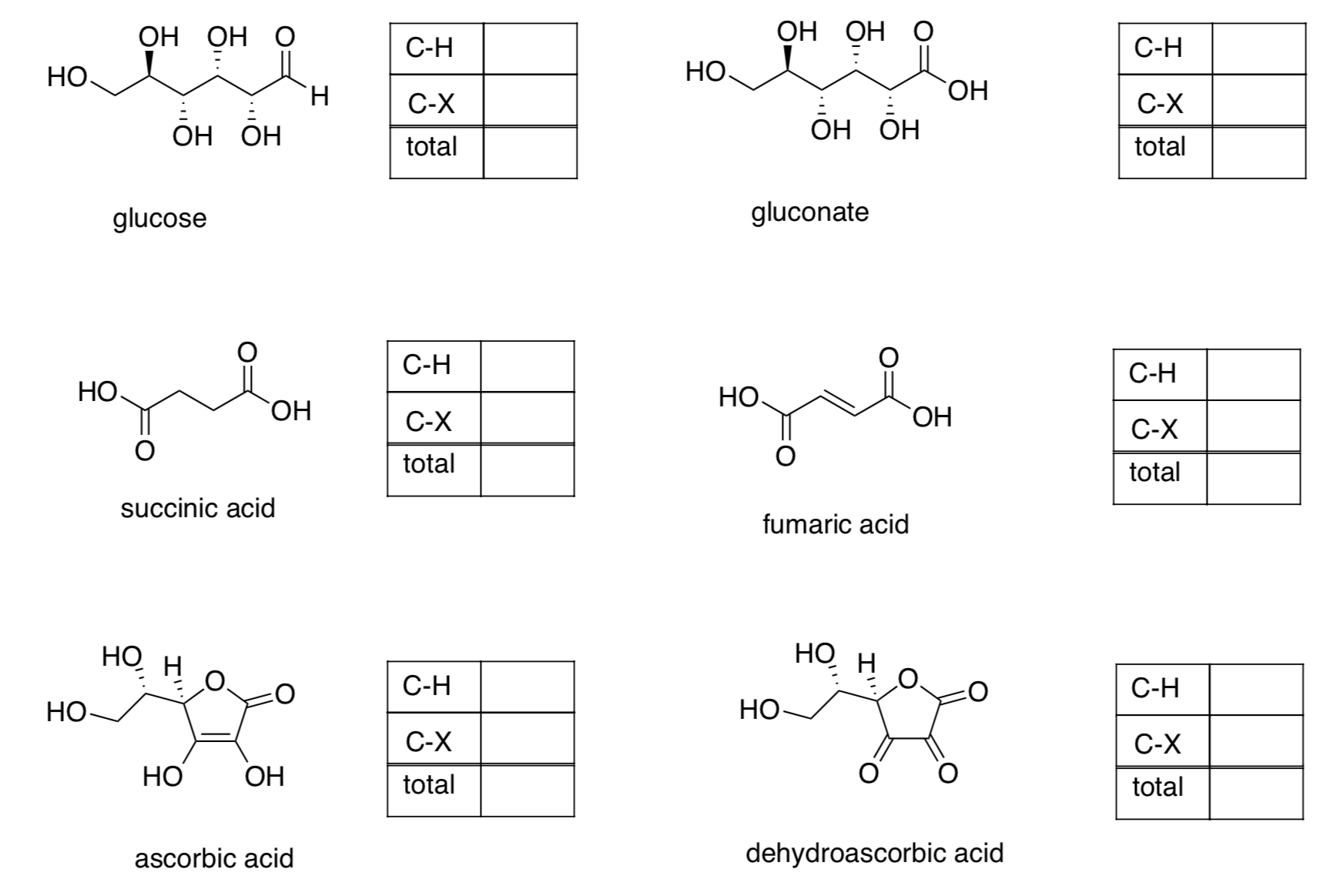
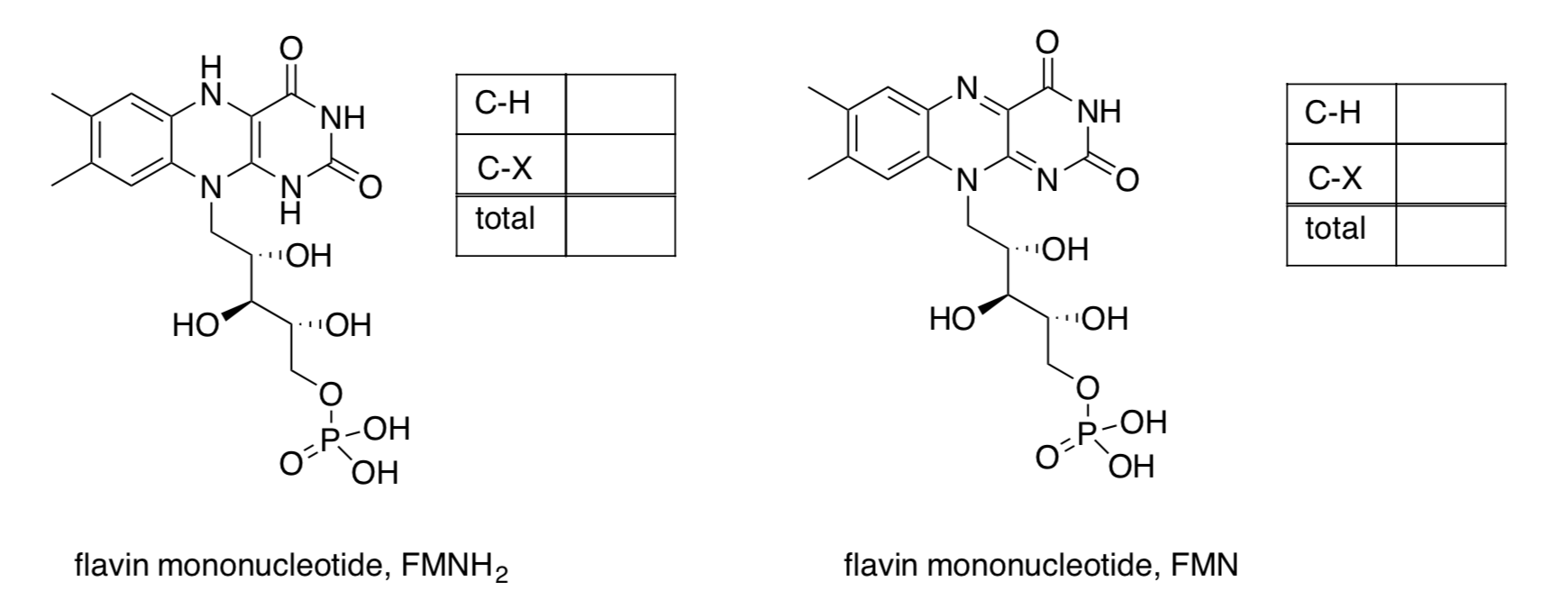
- In each case, add an arrow from reduced to oxidized cofactor and indicate the number of electrons provided.
Balancing Redox Equations
The half-reaction method for balancing redox reactions starts with splitting the reactions into the oxidation half-reaction and the reduction half-reaction. Each of the half-reactions are balanced independently then combined in such a way as to make the number of electrons lost equal the number of electrons gained.
Consider the redox reaction of Fe2+ with dichromate (Cr2O72-):
Fe2+ + Cr2O72- Fe3+ + Cr3+
Steps for Balancing Redox Reactions:
- Separate the unbalanced reaction into its two parts, oxidation and reduction
- Oxidation: Fe2+ -> Fe3+
- Reduction: Cr2O72- -> Cr3+
- Balance the two parts with regard to the atoms other than H and O
- Oxidation: Fe atoms are balanced
- Reduction: Cr2O72- -> 2 Cr3+
- Balance both half-reactions by adding water to balance the O.
- Oxidation: Fe2+ -> Fe3+
- Reduction: Cr2O72- -> 2 Cr3+ + 7 H2O
- Balance both half-reactions by adding H+ to balance the H.
- Oxidation: Fe2+ -> Fe3+
- Reduction: Cr2O72- + 14 H+ -> 2 Cr3+ + 7 H2O
- Balance both half-reactions for charge by adding e-.
- Oxidation: Fe2+ -> Fe3+ + 1 e-
- Left side +2, right side +3 so add one e- to right side
- Reduction: Cr2O72- +14H+ +6e- -> 2Cr3+ + 7H2O
- Left side +12, right side +6 so add 6 e- to left side
- Oxidation: Fe2+ -> Fe3+ + 1 e-
- Since the number of electrons that are given up in the oxidation half-reaction have to equal the number of electrons picked up by the reduction half- reaction, multiply one or both reactions so that the number of electrons lost will equal the number of electrons gained.
- Oxidation: 6 ( Fe2+ -> Fe3+ + 1 e-)
6Fe2+ -> 6Fe3+ +6e-
- Reduction: Cr2O72- +14H+ + 6e- -> 2Cr3+ + 7H2O
- Oxidation: 6 ( Fe2+ -> Fe3+ + 1 e-)
- Finally add the balanced half reactions together and cancel out the electrons and any other species common to both sides of the reaction.
6Fe2+ + Cr2O72- + 14H+ +6e- -> 6Fe3+ + 2Cr3+ + 7H2O + 6e-
6Fe2+ + Cr2O72- +14H+ -> 6Fe3+ + 2Cr3+ + 7H2O
balanced for acidic solution
- If the reaction is being done in a basic solution, add one OH- to each side of the equation for each H+, producing H2O on one side and excess OH- on the other.
6Fe2+ + Cr2O72- + 14H+ + 14OH- -> 6Fe3+ + 2Cr3+ + 7H2O + 14OH-
- Make any additional cancellations created by the formation of the new H2O molecules.
6Fe2+ + Cr2O72- + 14H2O -> 6Fe3+ + 2Cr3+ + 7H2O + 14OH-
6Fe2+ + Cr2O72- +7H2O -> 6Fe3+ +2Cr3+ +14OH-
balanced for basic solution
- Finally check the final reaction for charge and atom balance.
Balancing Reactions:
- Balance the following reactions that take place in aqueous acidic solutions.
- Cr2O72- + Cl- -> Cr3+ + Cl2
- Fe2+ + MnO4- -> Fe3+ + Mn2+
- Balance the following reaction in basic solutions
- CN- + MnO4- -> CNO- + MnO2
Practice Problems
- For each of following reactions:
- Determine the oxidation state of the atoms in the starting material.
- Determine the oxidation state of the atoms in the products.
- Determine which compound was oxidized/reduced/no change.
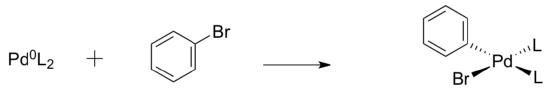
- CH2=CH2 + H2 + Pd -> CH3-CH3 + Pd
- Balance the following reactions that take place in aqueous acidic solutions.
- I- + IO3- -> I3- (what must be the product of both half reactions?)
- H2O2 + Fe2+ -> Fe3+ + H2O
- Cu +HNO3 -> Cu2+ + NO + H2O
- S2O32- + I2 -> I- + S4O62-
- Cr2O72- + C2O42- -> Cr3+ + CO2
- ClO3- + Cl- -> Cl2 + ClO2
- Mn2+ + BiO3- -> Bi3+ + MnO4-
- Balance the following reactions in basic solutions
- Br2 -> BrO3- + Br-
- Bi(OH)3 + SnO22- -> SnO32- + Bi
- When discussing the tricarboxylic acid cycle, many textbooks note that “the complete oxidation of glucose to CO2 involves the removal of 24 electrons.” Do an oxidation state calculation to confirm this analysis.


