7: Inner Sphere Electron Transfer
- Page ID
- 149277
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCIn the 1960’s, Henry Taube of Stanford proposed that halides (and other ligands) may increase the rate of electron transfer via bridging effects. Taube was awarded the Nobel Prize in chemistry in 1983.
- [Co(NH3)6]3+ + [Cr(H2O)6]2+ -> [Co(H2O)]2+ + [Cr(H2O)6]3+ + 6 NH3
k = 10-4 M-1 s-1
- [Co(NH3)5Cl]2+ + [Cr(H2O)6]2+ -> [Co(H2O)]2+ + [Cr(H2O)5Cl]2+ + 5 NH3
k = 6 x105 M-1 s-1
- Which reaction is faster? a. OR b.
- In each case, which metal is being oxidized?
- a.
- b.
- In each case, which metal is being reduced?
- a.
- b.
- Show, with curved arrows, the chloride ligand on Co3+ donating a lone pair to chromium, forming a bridge between cobalt and chromium (reaction b).
- Why can’t the ammine ligand (reaction a) promote inner sphere electron transfer?
After bridging, electron transfer could occur via electron movement to the bridging ligand or “hole movement” (i.e. electron is taken from ligand first and then replaced by an electron from the other metal).
- Show both possible mechanisms.
Substitution of one of the ammonias on the cobalt with alternate ligands reveals additional information.
- HCO2Co(NH3)53+ + Cr2+ -> Co2+ + Cr3+ + 6 NH3 k = 7.2 M-1s-1
- CH3CO2Co(NH3)53+ + Cr2+ -> Co2+ + Cr3+ + 6 NH3 k = 0.35 M-1s-1
- (CH3)3CCO2Co(NH3)53+ + Cr2+ -> Co2+ + Cr3+ + 6 NH3 k = 9.6x10-3 M-1s-1
- Draw structures for each of the bridging ligands.
- How do the structures of these ligands affect the ability of the bridging ligands to promote inner sphere electron transfer?
- Draw structures for each of the bridging ligands.
Summary
- Define Inner Sphere Electron Transfer:
- Define Outer Sphere Electron Transfer:
- What is the difference between the two mechanisms?
- Foran outer sphere electron transfer mechanism, which of these are true?
- Requires molecular collisions | T OR F
- Temperature dependent | T OR F
- Rate is dependent on solvent polarity | T OR F
- Rate is affected by steric hindrance | T OR F
- For an inner sphere electron transfer mechanism, which of these are true?
- Requires molecular collisions | T OR F
- Is dependent upon the type of ligands | T OR F
- Rate is affected by steric hindrance | T OR F
Inner Sphere/Outer Sphere Application Problems:
- The following data were gathered 20 years after the development of Marcus theory (Miller, JACS 1984, 3047).
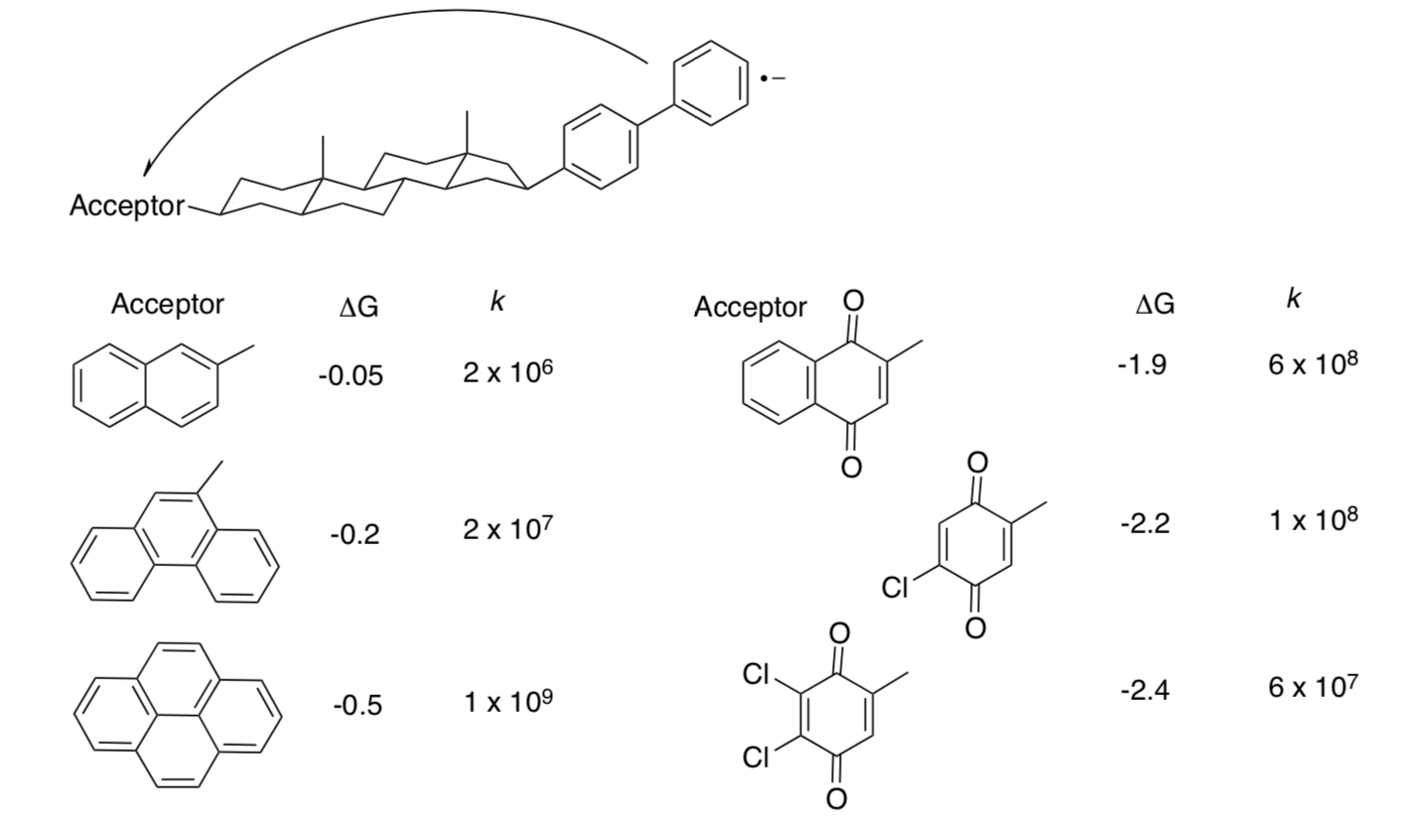
- Is this an outer sphere or inner sphere mechanism?
- Plot the data.
- Comment on their significance.
- Inner sphere and outer sphere electron transfer does not only occur between metals but can also occur between organic compounds. The following is taken from J. Am. Chem. Soc. 1999, 121, 617-626
The benzoquinone compounds below, in their photoactive state, were used as electron acceptors to monitor electron transfer from various aromatic donors by time-resolved laser flash experiments.
- Given the reduction potentials for the different benzoquinones, circle the stronger oxidizing agent.
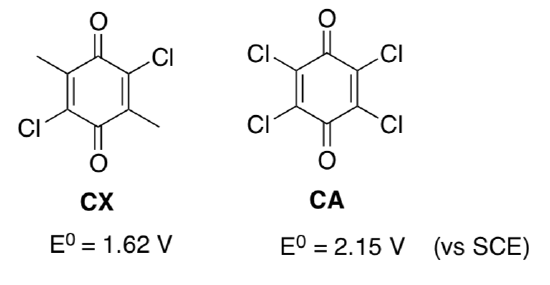
- Explain why there is a difference in reduction potential for the different benzoquinone compounds.
Researchers studied rates of electron transfer from the following aromatics to the quinones above.
rate constants k (106 M-1s-1) in different solvents redox couple CH3CN CH2Cl2 CHCl3 CCl4 HEB/CX
HMB/CX
TTB/CA
TMB/CA
970
5140
900
3900
160
5500
14
1200
40
2300
16
670
4
2300
0.3
250
The redox pairs above are selected so that they all have similar free energy of reaction (ΔGo), but the reaction rates still vary.
When examining how the pairs interact, researchers proposed a simple stacking model. An example of how the pairs stack is shown below:
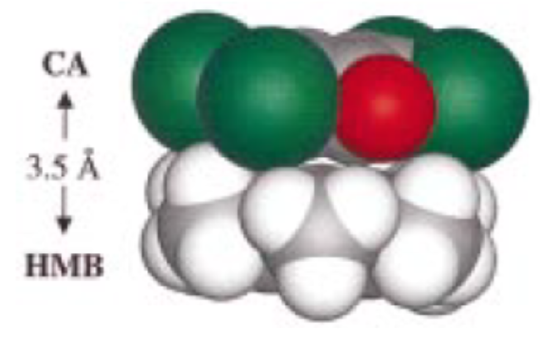
- Draw out how the TTB/CA pair might interact.
- Which pairs are more likely to react via an outer sphere mechanism? Why?
- Some of the reactions are much faster. What mechanism do they adopt?
- Compare the rates in acetonitrile to the rates in carbon tetrachloride (as ratios, e.g. 2x faster in acetonitrile). Which mechanism seems to be more sensitive to solvent polarity?
- Given the reduction potentials for the different benzoquinones, circle the stronger oxidizing agent.


