9: Battery applications
- Page ID
- 149477
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCRedox Applications: Building a Battery
Model 1: Mixing Everything in One Container
When a piece of zinc (Zn) metal is placed in aqueous Cu2+ the following reaction occurs spontaneously:
Zn(s) + Cu2+(aq) -> Zn2+(aq) + Cu(s)
Over time Cu(s) will deposit on the Zn strip and some of the solid zinc strip will dissolve.

Model 2: Separating the Half-Cells
A more useful approach is to separate the half-cells and put something in the middle like a light bulb or an iPhone. This is called a battery or voltaic cell. An example is:
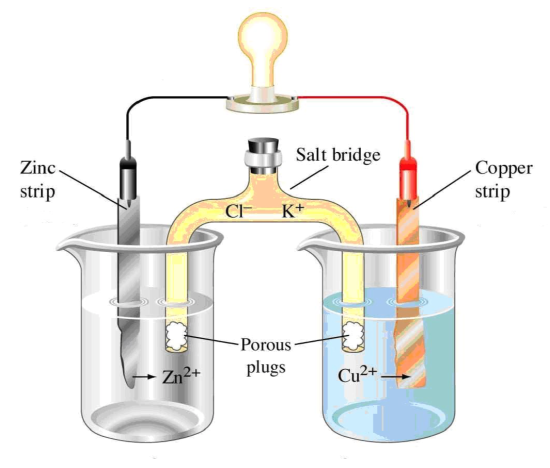
The same reaction occurs in Models 1 and 2 above:
Zn(s) + Cu2+(aq) -> Zn2+(aq) + Cu(s)
- Assuming that these reactions occur spontaneously, what is the sign of ΔG for the reaction?
- Write the half-reaction for the zinc electrode in Model 2 directly under the electrode in the figure. Is it an oxidation or a reduction?
- Write the half-reaction for the copper electrode in Model 2 directly under the electrode in the figure. Is it an oxidation or a reduction?
- Would the oxidation and reduction half-reactions you wrote for questions 2 and 3 be any different for Model 1?
- Model 2 is called a voltaic cell (a fancy term for battery), what advantage does it have over Model 1?
- Label the following parts or indicate the following on Model 2.
- Label the anode – the site of oxidation.
- Label the cathode – the site of reduction.
- Use an arrow to show which way electrons flow through the wire.
- Label the negative pole with a negative sign. (Hint: Electrons flow from negative to positive.)
- Label the positive pole with a positive sign. (Memory trick: Think of the ‘t’ in cathode as a plus sign.)
Voltaic cells are always set up the same way in terms of the anode and cathode.
- Fill in the blanks below:
The anode is always on the __________ (left, right) and that is where ___________ (oxidation, reduction) occurs. The anode is also the ____________ (positive, negative) pole of a battery.
The salt bridge in Model 2 is very important since solutions cannot be charged.
- For example, what ion is being made at the anode in Model 2?
- Will the water near the anode become positive or negative?
- If the salt bridge contained KCl(aq), which of the ions in KCl would flow to the anode to balance the charge that is being made?
- Draw arrows on the salt bridge showing which direction the K+ and Cl- move.
Model 3: Shorthand Notation
Drawing out voltaic cells like Model 2 can be cumbersome, so a shorthand notation has been developed. The shorthand notation example below is a compact version of the voltaic cell shown in Model 2.

- Write the chemical equation that correlates to:
Na(s)|Na+(aq)||Ag+(aq)|Ag(s)
- Write the shorthand notation for the following reaction. Note that the equation does not need to be balanced to do the shorthand notation.
H+(aq) + Al(s) -> Al3+(aq) + H2(g)
Batteries We Use
Car Battery
A car battery utilizes two reactions that involve lead (Pb).

One unique aspect is that one of the products, PbSO4, is the same for each reaction.
- What happens to the charge on lead during the anode reaction?
- What happens to the charge on lead during the cathode reaction?
- Use E°cell = E°cathode – E°anode to determine the voltage for a car battery.
- Calculate the voltage for a car battery using this approach:
- Add the reduction potentials for the two half-reactions.

- Add the reduction potentials for the two half-reactions.
- A typical car battery is a series of cells with a total of 12 Volts. How many cells are needed to get close to 12 Volts?
Application Problems
- The energizer battery uses this following set of reactions:

- Determine the voltage for an Energizer.
- Label the anode, the cathode, and use an arrow to show which way the electrons flow in the somewhat cheesy diagram.

- A “dental voltaic cell” is created when a person chews on aluminum foil and it comes into contact with a metal filling. Pain results if the flow of electrons encounters a nerve ending. If you have chewed a piece of aluminum foil you know the sensation.

- Write the overall balanced equation and identify the species that is oxidized and the one that is reduced.
- Use the table of reduction potentials in your text to determine the voltage that is transferred to a person’s nerve ending.
- Which would cause a bigger jolt of pain: aluminum foil or tin foil? Briefly explain your answer. (Hint: Consult a table of reduction potentials.)
- A voltaic cell is made as shown by the shorthand notation below.
- Balance the reaction in aqueous acidic solution.
MnO2(s)|MnO4-(aq)||F2(g)|F-(aq)
- Balance the reaction in aqueous acidic solution.
- In a battery, an oxidation half-reaction is physically separated from a reduction half-reaction so that the electrons are transferred “through the wires” to do useful work instead of causing a direct reaction.
- Using the table of Standard Reduction Potentials, what should be the
maximum voltage obtainable from a single battery cell? What would be the overall reaction occurring in this battery? Why do you suppose there are no practical applications for this battery?
- A lithium ion battery has a voltage of about 3V. It can be shown that ΔGo = - nFEo, where n is the number of electrons transferred in the balanced equations (this is one in a lithium battery), F is the Faraday constant (96,500 joules/Volt). Calculate ΔGo for the reaction in kJ. For a chemical reaction is this free energy change relatively large or small? As equilibrium will it be mostly products, reactants or a mixture of both?
- What, chemically, happens when a battery is “dead”? What, chemically, happens when a battery is recharged?
- Using the table of Standard Reduction Potentials, what should be the


