22: Photochemical Hydrogen Production
- Page ID
- 150978
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCTowards a Hydrogen Economy
Our economy depends on the release of energy when petroleum is burned. The carbon dioxide produced contributes significantly to the greenhouse effect. In contrast, burning hydrogen produces water, which should have less environmental impact.
- Write a reaction for the combustion of hydrogen.
- The energy released when H2 is burned: -118.5 kJ/mol). Is that [ more or less ] efficient than gasoline (44 kJ/g) or ethanol (29 kJ/g)?
The formation of H2 is endothermic. Industrially, hydrogen is often produced by steam reforming of methane (from natural gas).
CH4 +H2O→CO+3H2
- Where does the energy to make H2 come from?
- Do you think making H2 from a process driven by petroleum is a good idea? Explain.
A hydrogen economy will require an inexpensive and green source of hydrogen. In photocatalysis, light from the sun would be used to provide the energy for this process, and we have plenty of free sunlight.
Photochemical Hydrogen Production
Eisenberg, Holland & Krauss at University of Rochester recently reported that CdSe nanoparticles can act as photocatalysts for nickel catalyzed H2 production from water. (Science DOI 10.1126/science.1227775)
- Complete the following catalytic cycle for the H2 production.
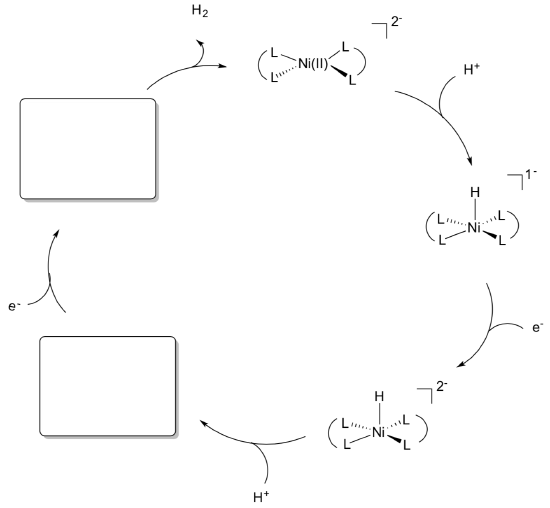
The nickel catalyst is a square planar complex formed from 2 bidentate DHLA-3 ligands.
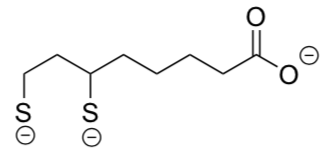
- Draw this complex.
The researcher noted that it had been previously found that DHLA2- binds to Ni(II) with a K of approximately 1010.
- Suggest two reasons (one based on entropy and one based on enthalpy) why the K for complex formation is large.
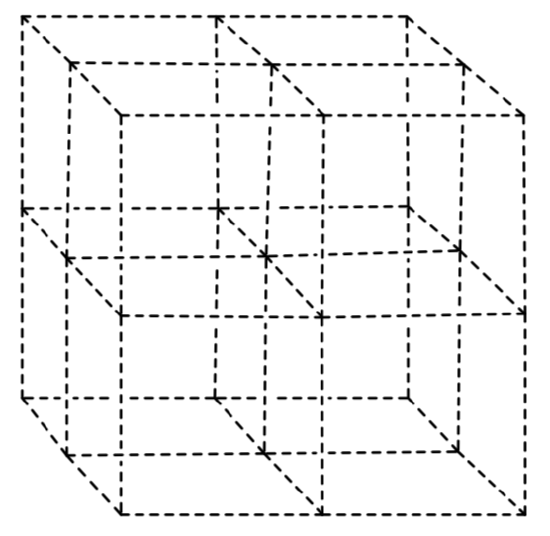
Electrons are donated into the nickel catalyst from CdSe nanoparticles. These are very small chunk of a crystal lattice. The crystal structure of CdSe consists of a face- centered cubic array of selenium ions with cadmiums in one-half of the tetrahedral holes.
- Draw that structure on the framework below.
- Confirm that your structure has the proper empirical formula.
In order to prevent the nanoparticles from condensing into larger crystals, the surface of the CdSe nanoparticle was coated with dihydrolipoic acid (DHLA, below).
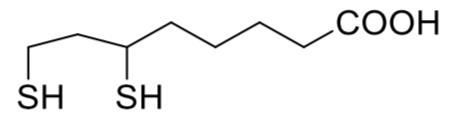
- Draw some atoms in the following cartoon of a small CdSe nanocrystal and show how several DHLA molecules interact with specific atoms on the surface.

- How will DHLA help keep the nanoparticle suspended in water?
- How will the DHLA help keep the nanoparticles from coalescing into a “non-nano” particle?
The Photocatalytic System
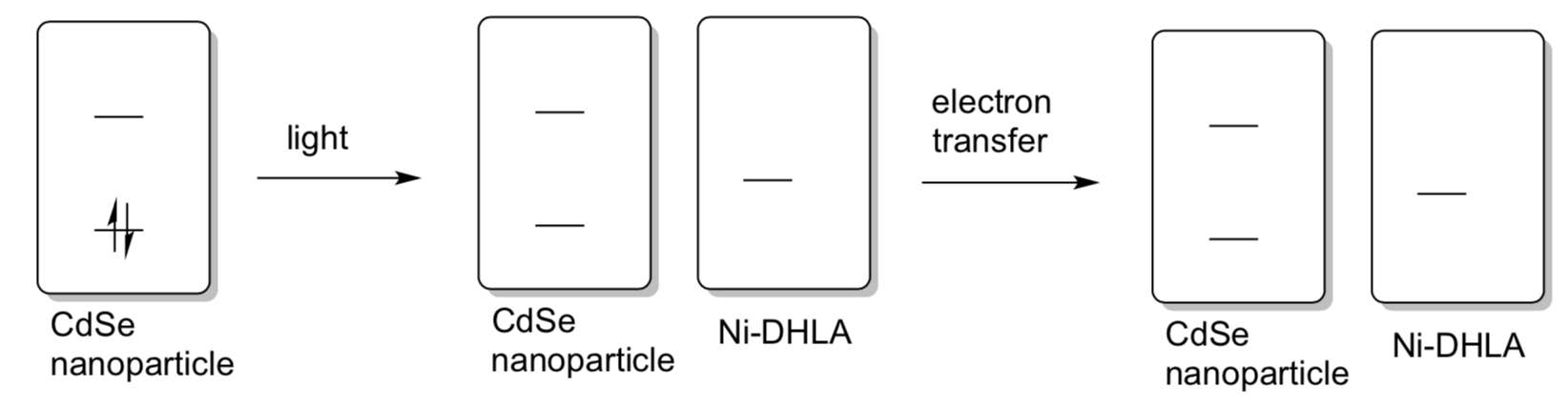
Eisenberg’s CdSe nanoparticles can absorb light and then transfer that higher energy electron to the Ni-DHLA for hydrogen production.
- Complete the following simplified MO diagram to show this process.
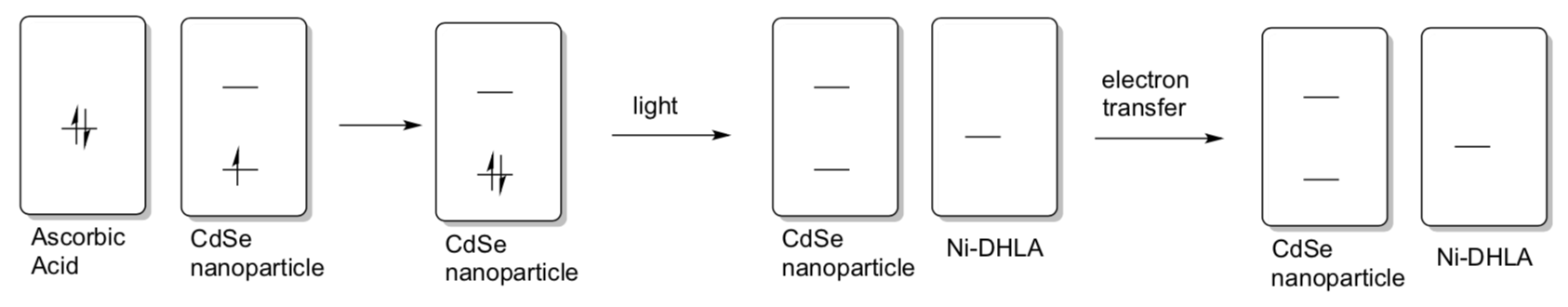
Since we have removed an electron from CdSe to do the reduction, we need to replace it in order to achieve catalysis. In this case researchers used ascorbic acid (vitamin C) as a sacrificial 2-electron donor. It forms dehydroascorbic acid and releases two protons as it adds electrons to the CdSe.
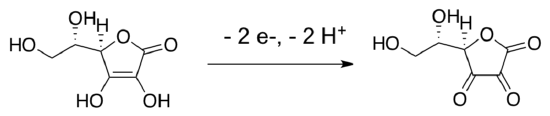
- Show a mechanism with intermediates for this conversion. Keep in mind that each CdSe “unit” is accepting a single electron.
- Show the transfer of these electrons in your CdSe MO diagram.
Sizes of Nanoparticles
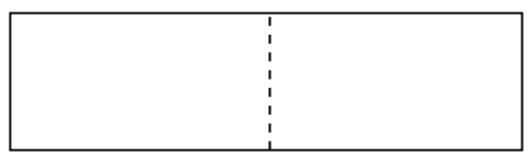
Eisenberg and colleagues report that CdSe nanoparticles were prepared with diameters ranging from 2.5 to 5.5 nm. These sizes were determined from the wavelength of light absorbed. Light absorption by nanoparticles is based on the principles of “a particle in a box”. The longer the box, the longer the wavelength of an electron in the box.
- In the following box, draw in the longest possible wave so that an entire wavelength fits inside the box and the node falls on the dashed line.
This wave has an energy described by the Planck-Einstein relationship, E = hc/l. Call that energy “U” for “unit of energy”.
- Add in the second-longest possible wavelength in the box.
- What is its energy, compared to U?
- What is the difference between the energies of the two waves? If a photon were absorbed by this nanoparticle, how much energy would it need to have?
- Add the wavelength for the lowest-energy electron to this larger box (nanoparticle).
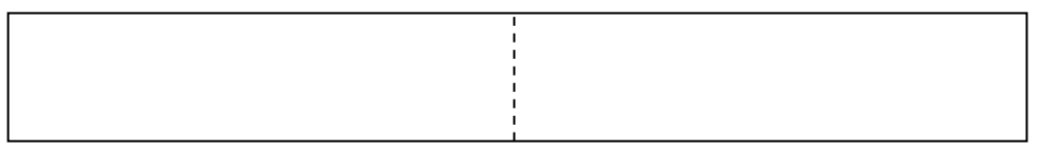
- What is its energy, compared to U?
- Add in the second-longest possible wavelength in the box.
- What is its energy, compared to U?
- What is the difference between the energies of the two waves? If a photon were absorbed by this nanoparticle, how much energy would it need to have?
- In general, how is the size of a nanoparticle related to the energy of a photon absorbed? To the wavelength of photon?
The Rochester team could prepare CdSe nanoparticles that absorbed light with maxima at 520, 540, 570, 600 and 620 nm.
- What is the λmax of the 2.5 nm particle?
- What is the λmax of the 5.5 nm particle?
H2 Evolution?
Eisenberg and co-workers shone light on the sample and waited for H2 to evolve. Their results are shown below.
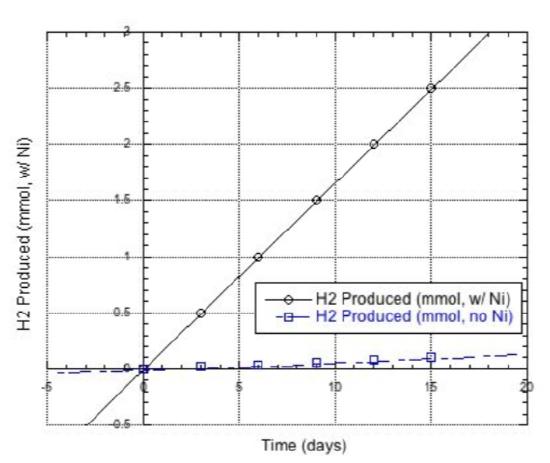
- What did they conclude? Can CdSe nanocrystals convert water to H2?
If, after conducting a hydrolysis experiment (H2 production from water), the CdSe nanoparticles were centrifuged, filtered and rinsed, catalytic activity was completely lost.
- What does this fact tell you about the location of the nickel complexes: in solution or on the nanoparticle surface?
- Does this process probably involve inner sphere or outer sphere electron transfer?
Optimizing H2 Evolution
Each of the different sizes of nanoparticles was assayed for its ability to provide an electron in this process. The results are shown below. Note that the concentrations used in this study (4 μM) are different from the previous graph (1 μM).
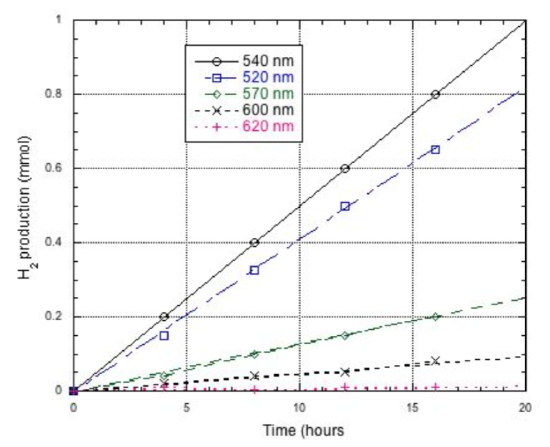
- Why do different nanoparticles have different activity levels?
The paper notes that the optimal system produces 600,000 moles H2 per mole of catalyst after 110 hours.
- If this rate is constant, how many H2’s per mole of catalyst are produced per second (i.e. what is the turnover frequency)?
Other photocatalysts that form hydrogen have been found;; however they tend to not last very long because the “dye” that receives electrons from the excited state is said to “bleach” (decompose).
- If it bleaches, what kind of reaction does it undergo? (What does bleach (NaOCl) do?)
- Do you think this system will undergo similar bleaching? Why or why not?


