23: Photoredox Catalysis
- Page ID
- 150979
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
α-Alkylation: HOMO and LUMO Catalysis
In a related set of reactions, MacMillan’s group (Princeton) has used enamine and iminiumcatalysis to effect chiral alkylations.
Fill in the products to show the catalysts.
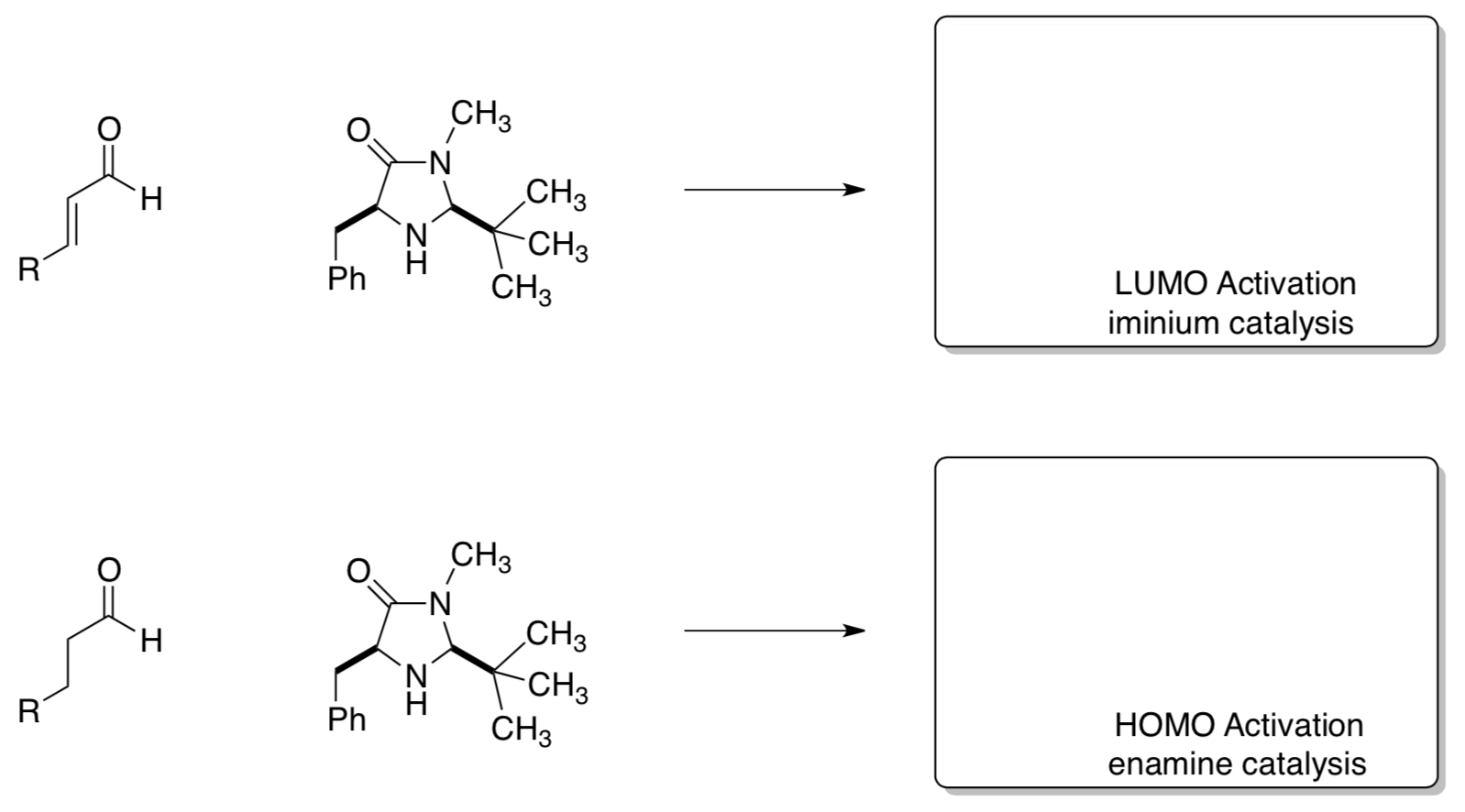
- Show how these two catalysts can rapidly interconvert (enamine has four π electrons and iminium has two π electrons).
- The chiral iminium acts as a(n) electrophile OR nucleophile (circle one), these reactions are referred to as LUMO activation.
- Circle the activated site.
- The chiral enamine acts as a(n) electrophile OR nucleophile (circle one), these reactions are referred to HOMO activation.
- Circle the activated site.
- Show how the HOMO activated catalyst will react with CH3I to form a chiral center. Draw the product clearly. Is the product (R or S)?
SOMO Catalysis
The MacMillan group decided to try a one-electron oxidation of a transient enamine species (shown below) to generate a three p electron radical cation with a singly occupied molecular orbital (SOMO) that is activated toward a range of enantioselective catalytic transformations not currently possible with established catalysis concepts.Beeson, Mastracchio, Hong, Ashton and MacMillan, Science, 2007, 316, 582-585.
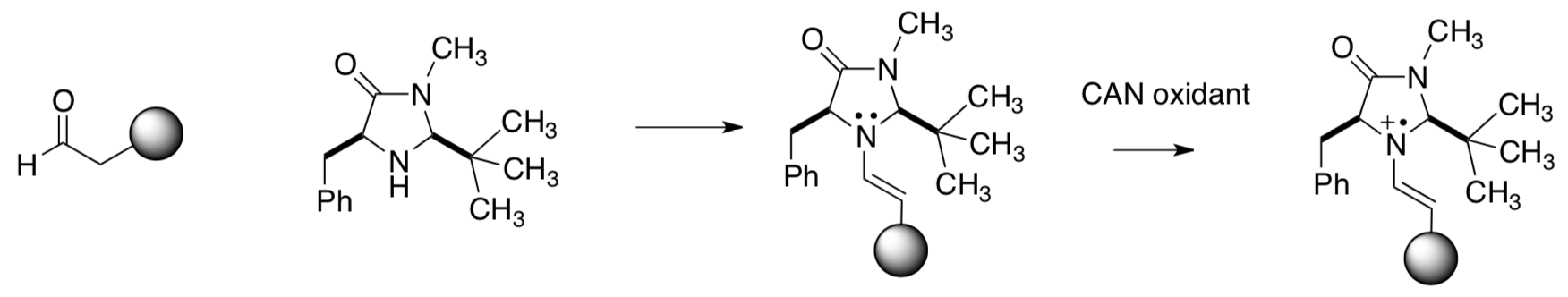
Formation of the radical cation
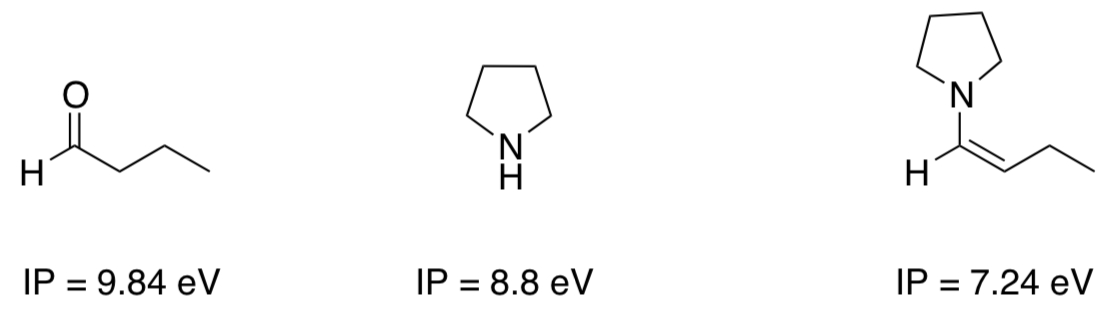
- If there are three compounds in solution (aldehyde, amine and enamine), which one will be oxidized? Circle the one that is most likely to be oxidized.
- CAN oxidant: ceric ammonium nitrate
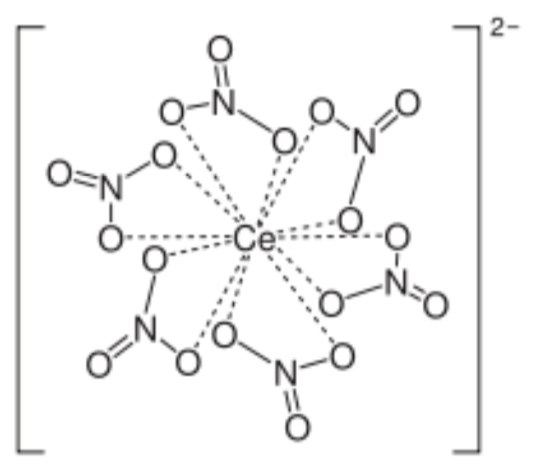
- What is the coordination number?
- Why is the CN so high? Hint: Look at its location on the periodic table.
- What is the charge on the cerium?
- When it reacts with the enamine, it accepts/loses electrons (circle one).
- What will be the new charge on Ce?
SOMO Aldehyde Alkylation Catalytic Cycle
- Fill in the missing products in the following catalytic cycle for SOMO activation.
- Draw arrows for these conversions.
- Write out the generalized reaction (like we usually put in the box in the corner).
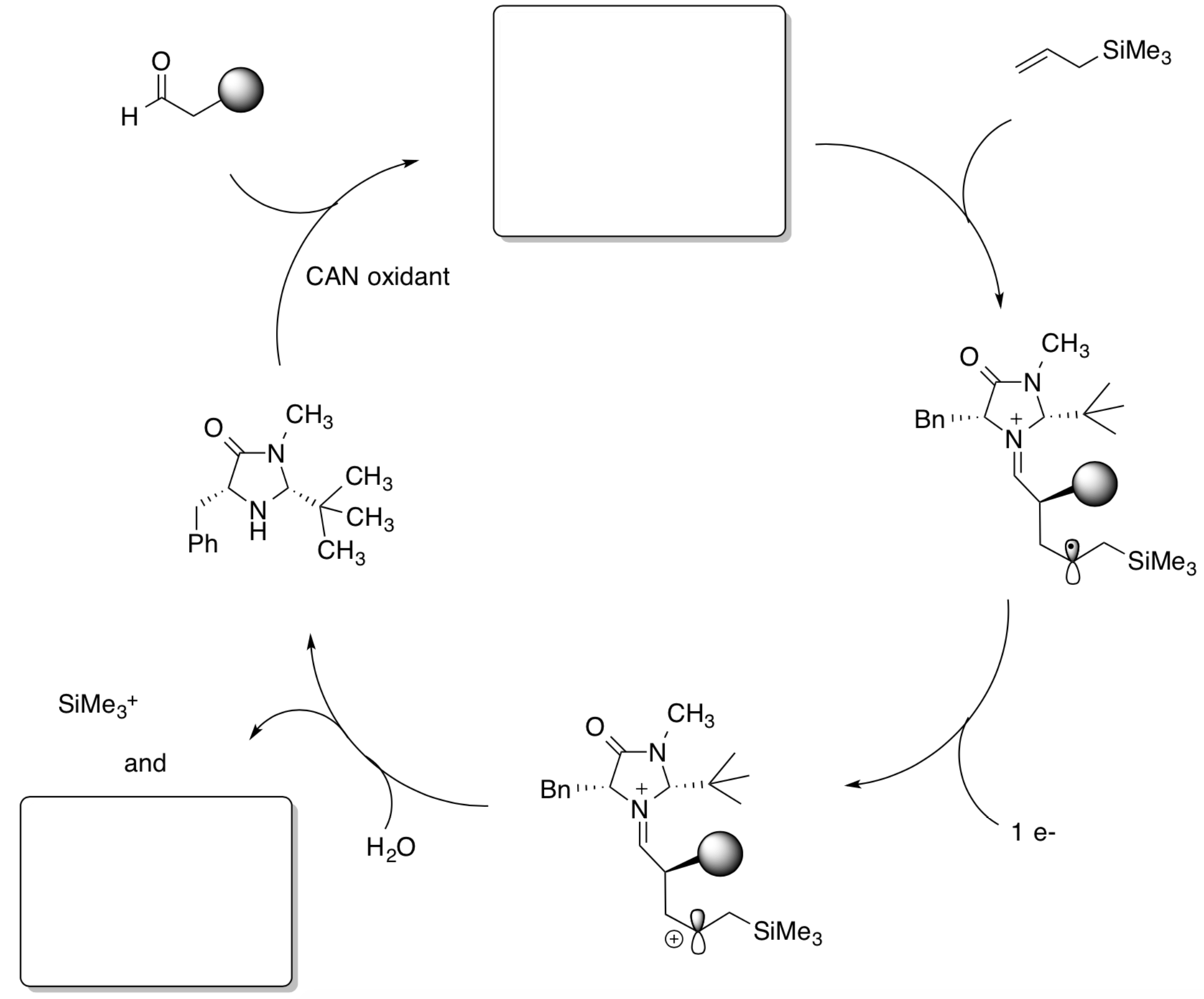
Stereocontrol: Activated Face
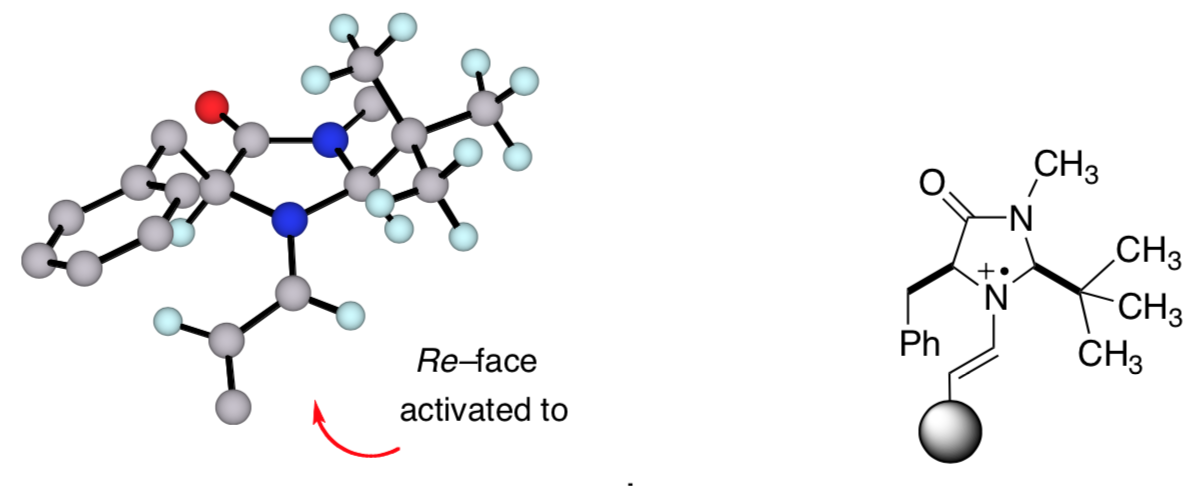
Using these pictures of the SOMO catalyst:
- Identify the reactive carbon.
- Draw a resonance structure of the catalyst with a radical on the carbon that forms the new bond.
Typically, carbocation and radical intermediates result in racemic mixtures for the products.
- What is the geometry of a carbocation?
- What is the geometry of a radical?
- Explain (using pictures) why radicals and carbocations usually proceed with racemization.
- Why does this catalyst result in a chiral product even though there is a radical intermediate?
- The product always forms on the Re face of the alkene (ie leading to the R product). Show how this leads to an R enantiomer.
Application Problem:
- HowdidMacMIllandeterminethatSOMOcatalysisproceededthrougharadical intermediate?
Beeson, Mastracchio, Hong, Ashton and MacMillan, Science, 2007, 316, 582-585.
- Label these intermediates with HOMO or SOMO catalysis.
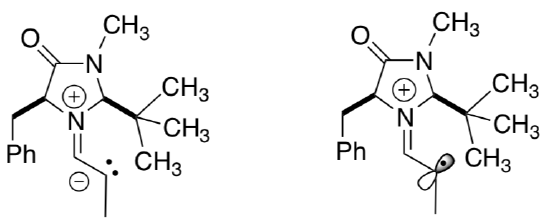
- SOMO vs. HOMO reactivity: radical clock
If HOMO:
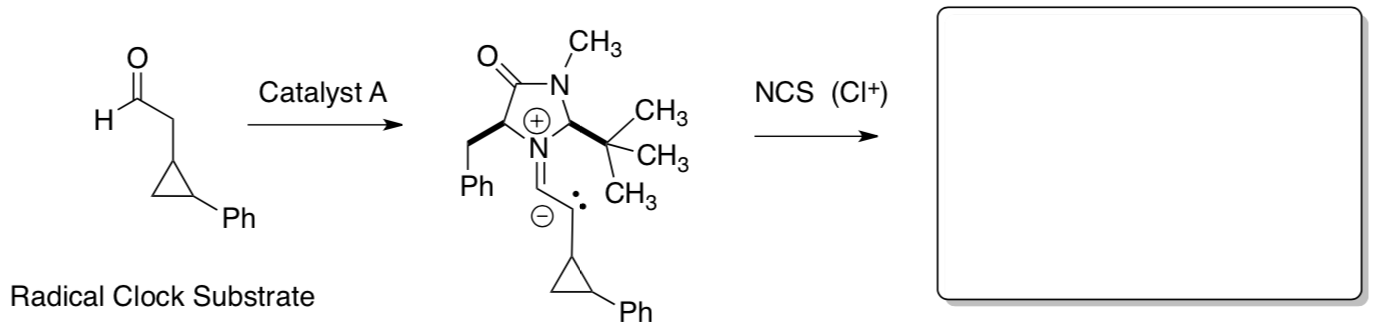
If SOMO:
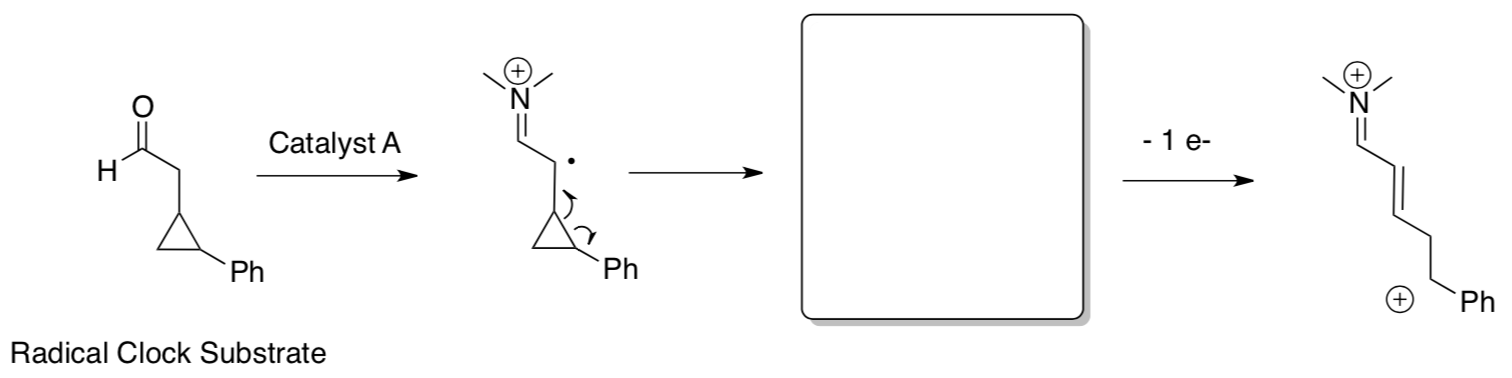
Experimental Data:
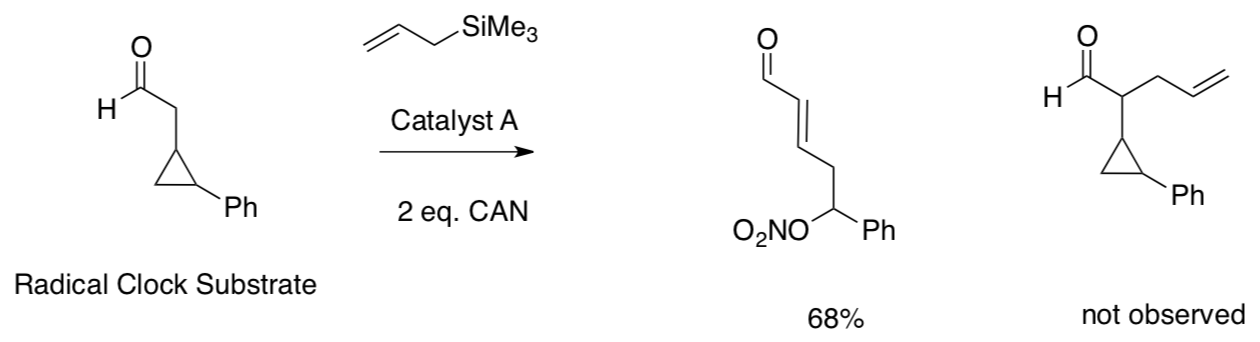
- Show how these products were formed.
- What does the experimental data reveal about the mechanism?
- Label these intermediates with HOMO or SOMO catalysis.
- ApplicationtoNaturalProductSynthesis:
Jones, Simmons, Mastracchio and MacMillan, Nature, 2011, 475, 183-188.In nature, specific enzymes in catalytic cascades rapidly produce biosynthetic intermediates which are modified to form a range of structurally related members of the same natural product family. For example, the following six biologically active alkaloids probably are derived from a common metabolite, tryptamine.
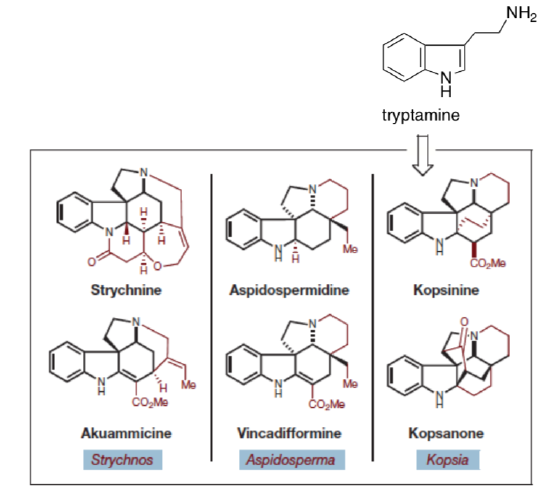
In a biomimetic strategy, MacMillan’s group developed the following synthesis of a common synthetic intermediate to be used in the syntheses of these alkaloids.
- Draw arrows for these conversions that occur in one pot.
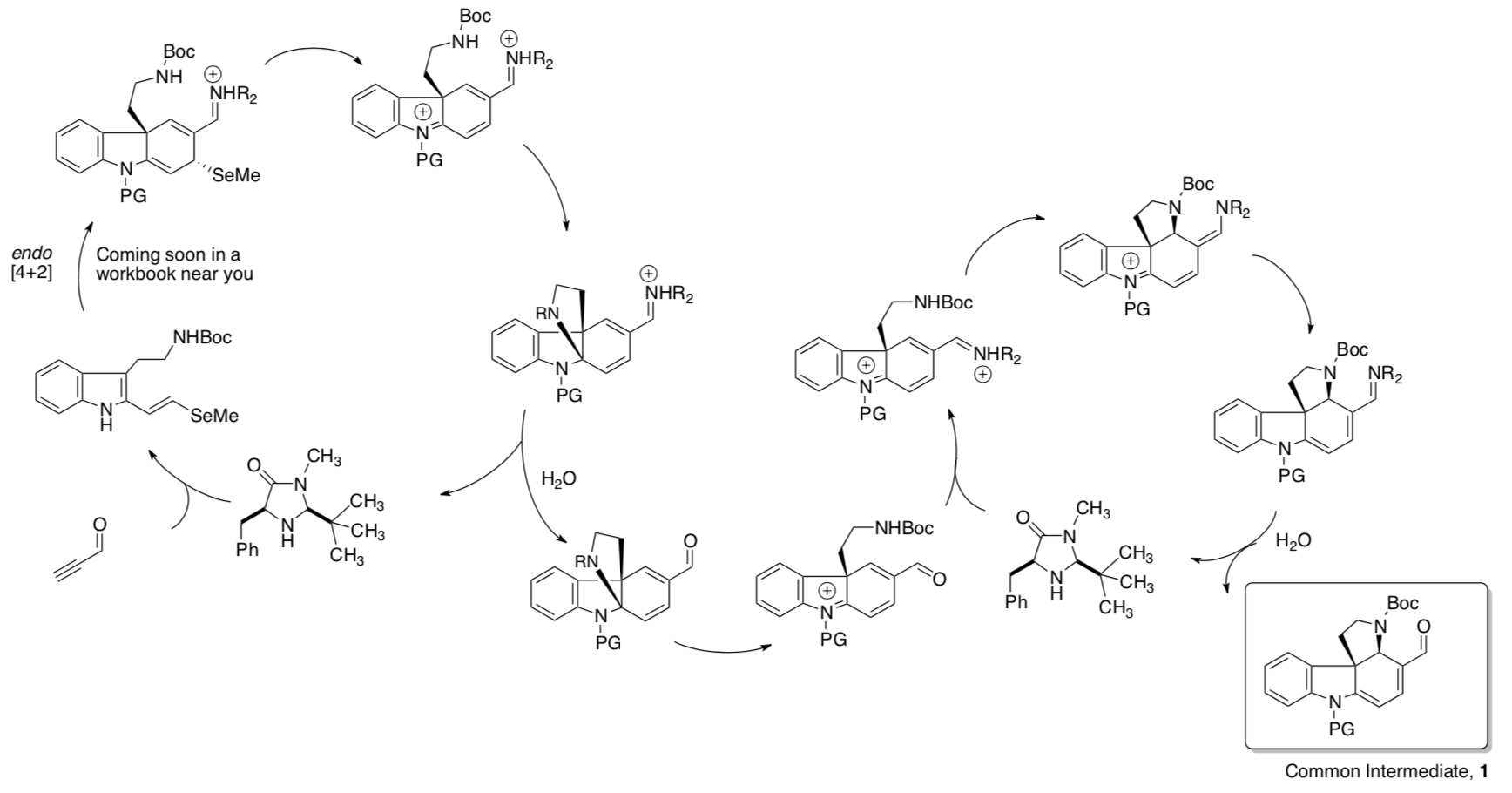
Common Intermediate 1 can be converted to different alkaloids.
- Fill in the boxes with the reagents.
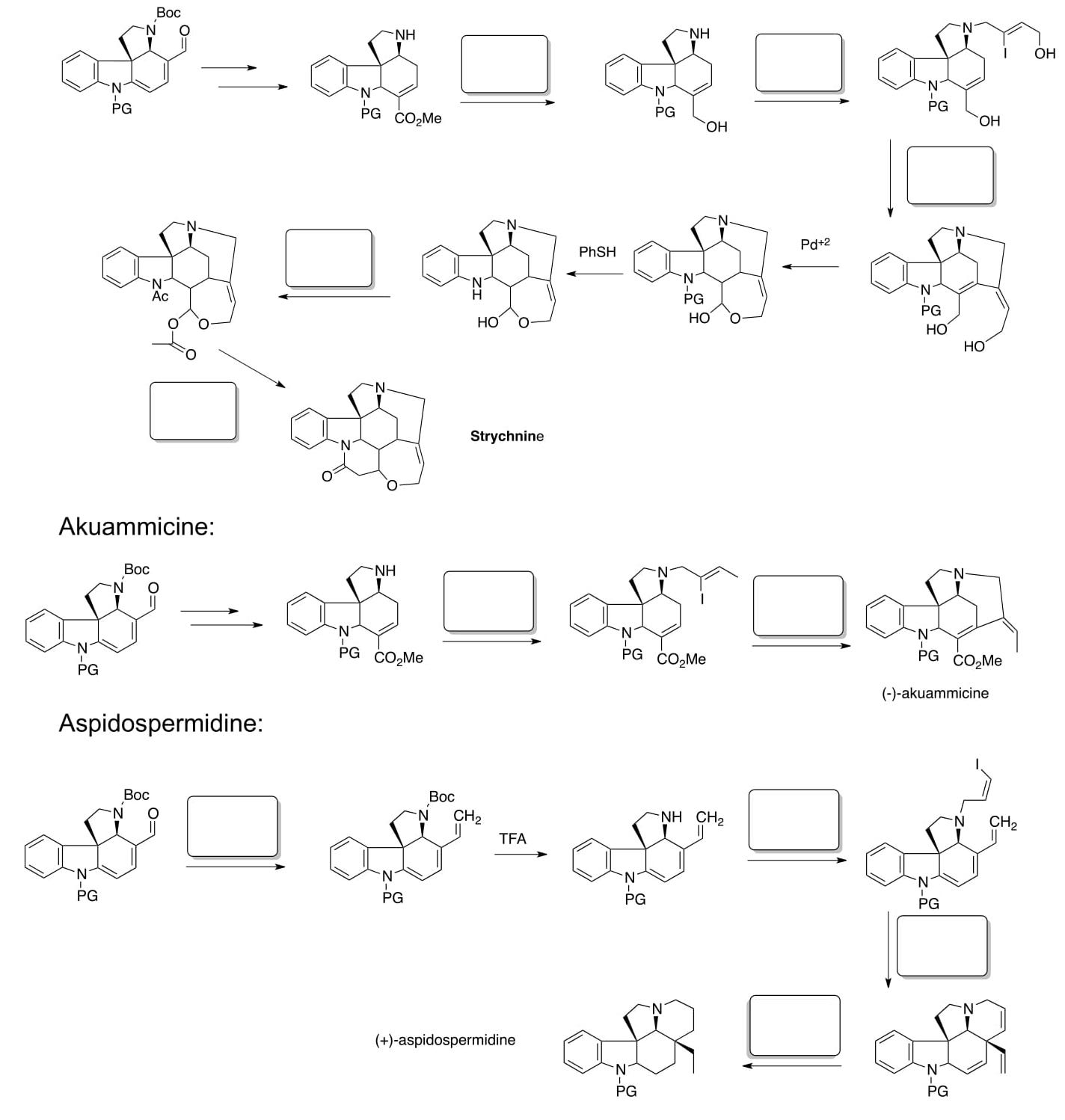
Extra Credit: Create a Road Map (using ChemDraw) for kopsinine and kopsinone from this same paper.
- Draw arrows for these conversions that occur in one pot.
- BiomimeticSynthesisofSteroids
Biomimetic synthesis of steroidal systems from acyclic polyenes has long been an area of great interest. MacMillan developed these syntheses using his SOMO catalysis.
Rendler & MacMillan, J. Am. Chem. Soc. 2010, 132, 5027.
- The oxidizer is different than CAN. Circle the oxidizer in this reaction below.
- What is the oxidation state of the Cu at the beginning of this reaction?
- What would you predict the oxidation state of Cu to be at the end of the reaction?
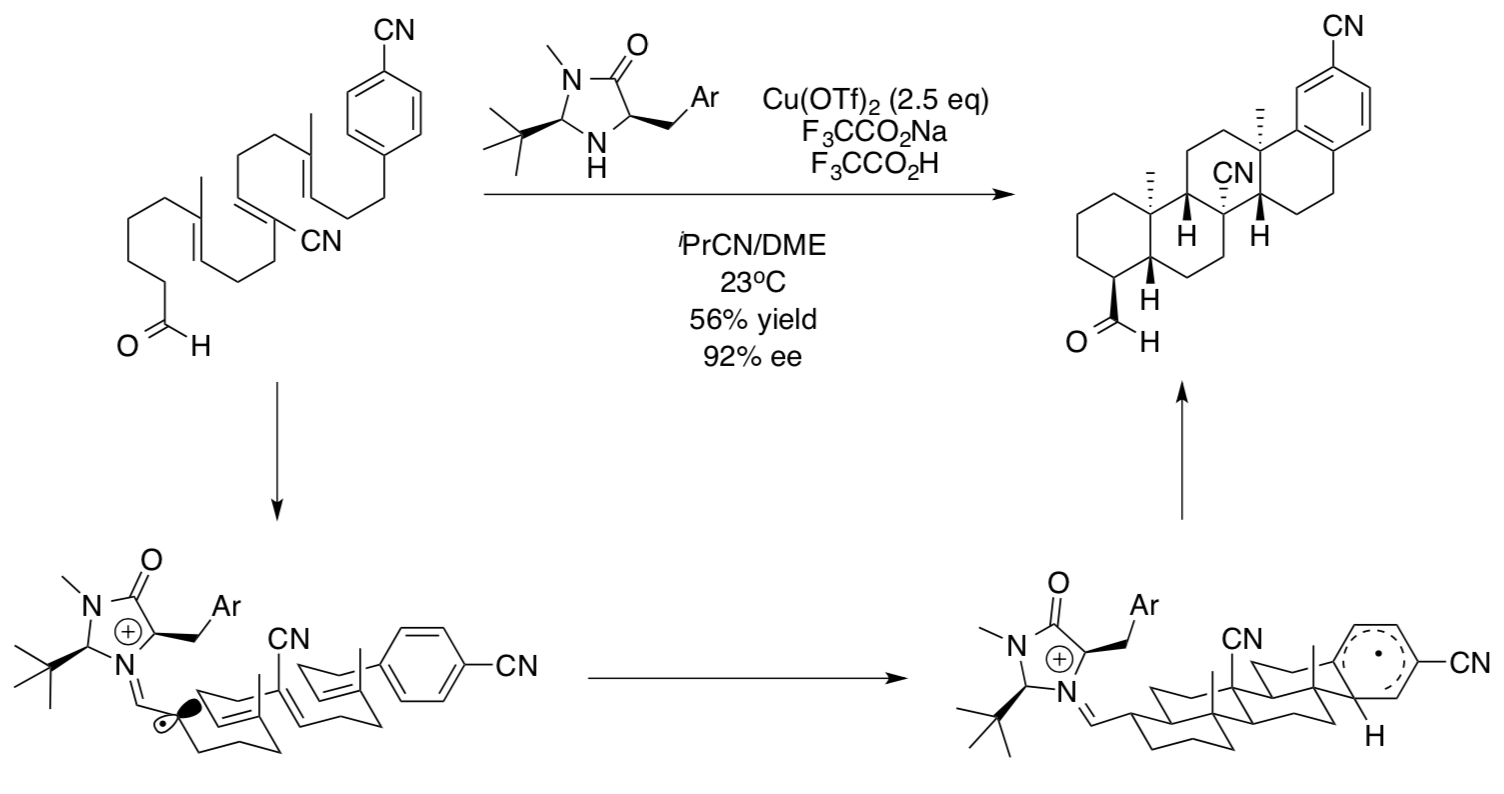
- Highlight the new σ bonds in the product.
- Use the first reaction intermediate to illustrate the flow of electrons in this cyclization mechanism.
- Draw in the π* orbitals of the alkene and aryl groups to show how they overlap to form new s bonds.
- Predict the products for the reactions below:
[NB: These reactions have a 2:1 to 4:1 preference for ortho selectivity]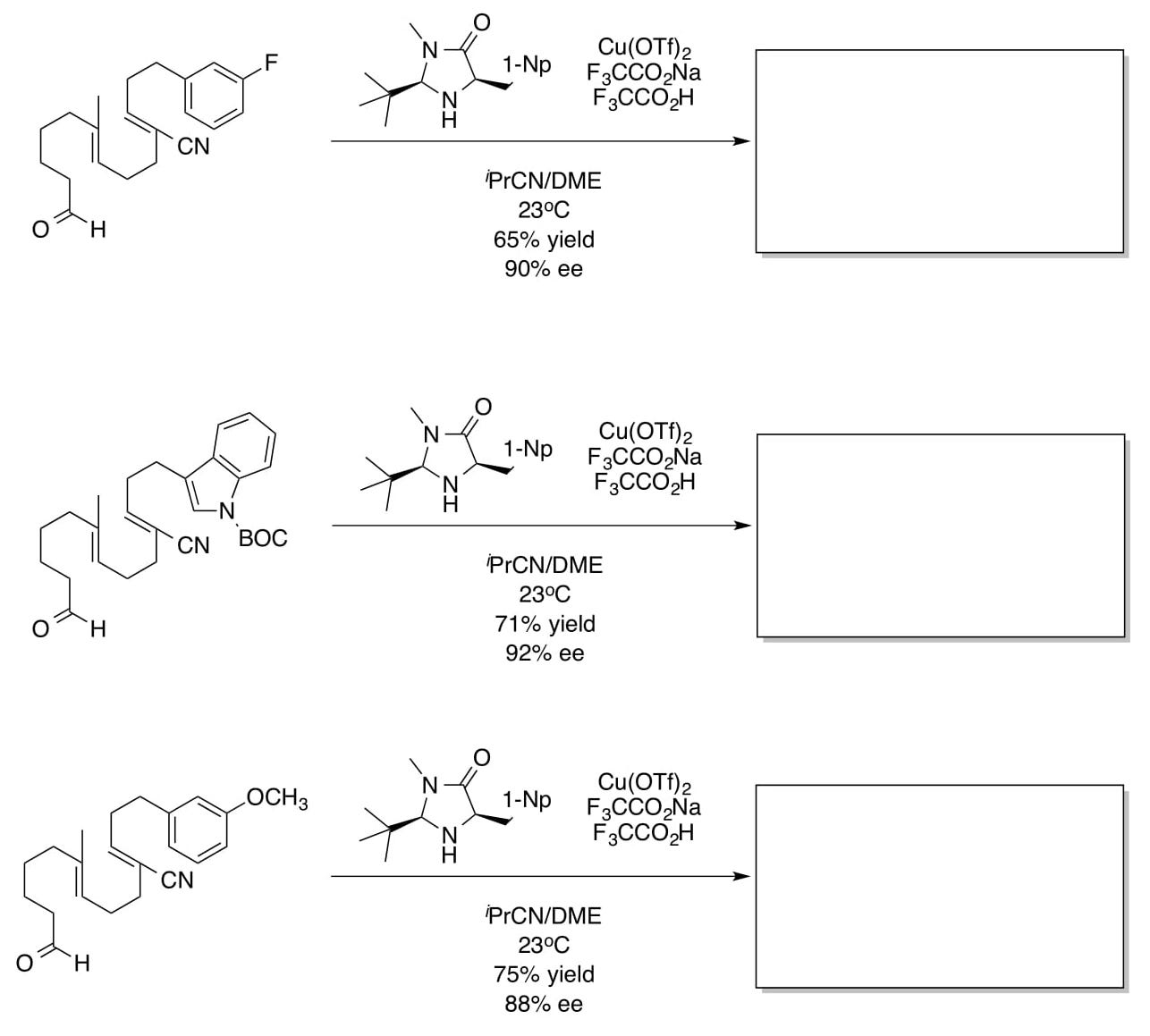
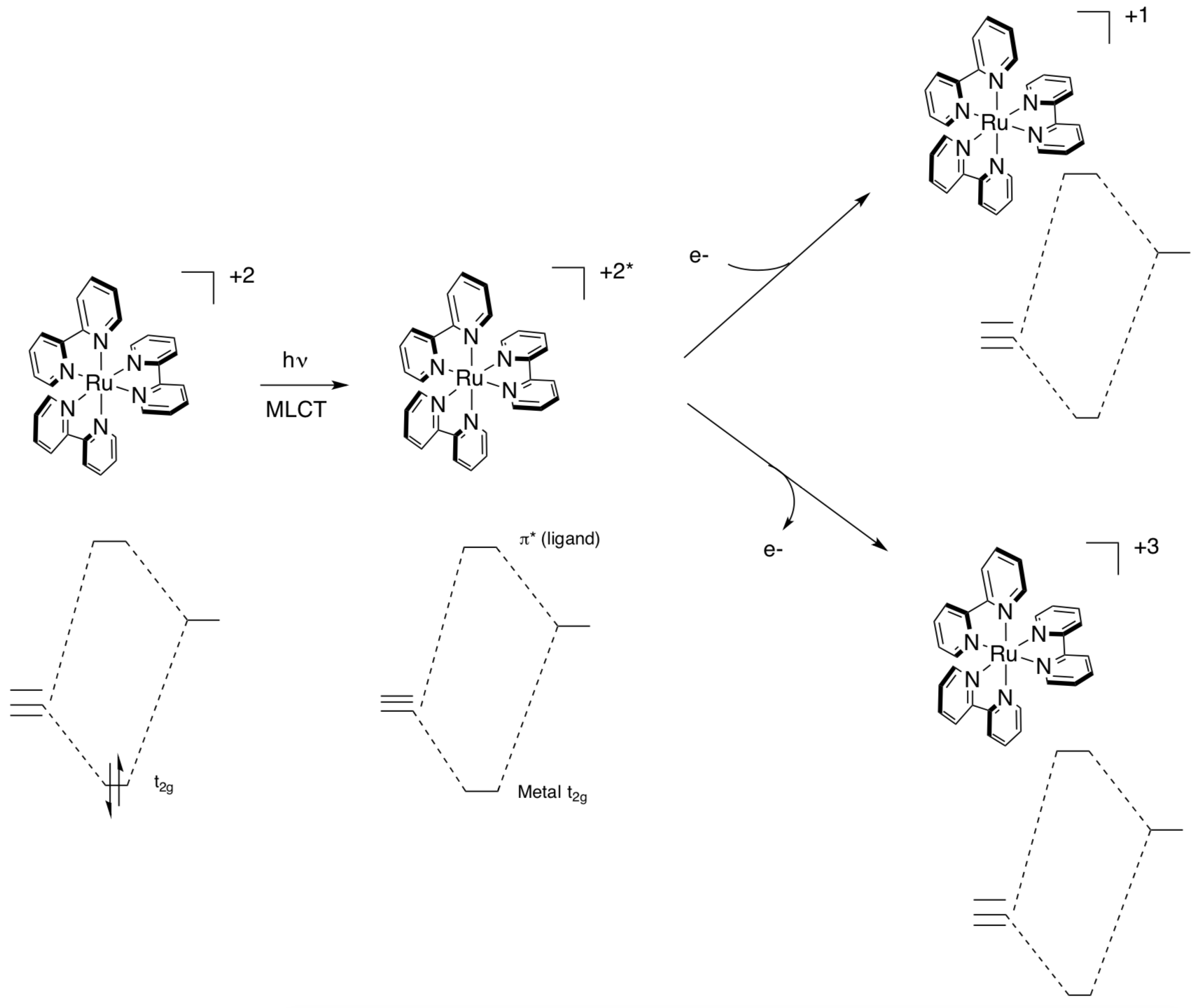
Photoredox Catalysis
In the previous SOMO catalytic cycle, two equivalents of the CAN oxidant are needed which generates a lot of waste. There is a need for an alternate radical initiator.
Nicewicz and MacMillan used the approach to use Ru(bipy)3 and light.
Nicewicz and MacMillan, Science, 2008, 322 (5898), 77- 80.
[Ru(bipy)3]2+ (shown below) absorbs UV light and visible light. The triplet excited state has both oxidizing and reducing properties. This unusual situation arises because the excited state can be described as an Ru3+ complex containing a bipy- ligand.
- Fill in the electrons for the +1 oxidation state.
- Fill in the electrons for the +3 oxidation state.
- Label the two Ru+2 sites as singlet or triplet states.
Photoredox Catalytic Cycle WITH SOMO Catalysis
- Fill in the missing products in the following catalytic cycle for SOMO activation.
- Draw arrows for these conversions.
- Write out the generalized reaction (like we usually put in the box in the corner).
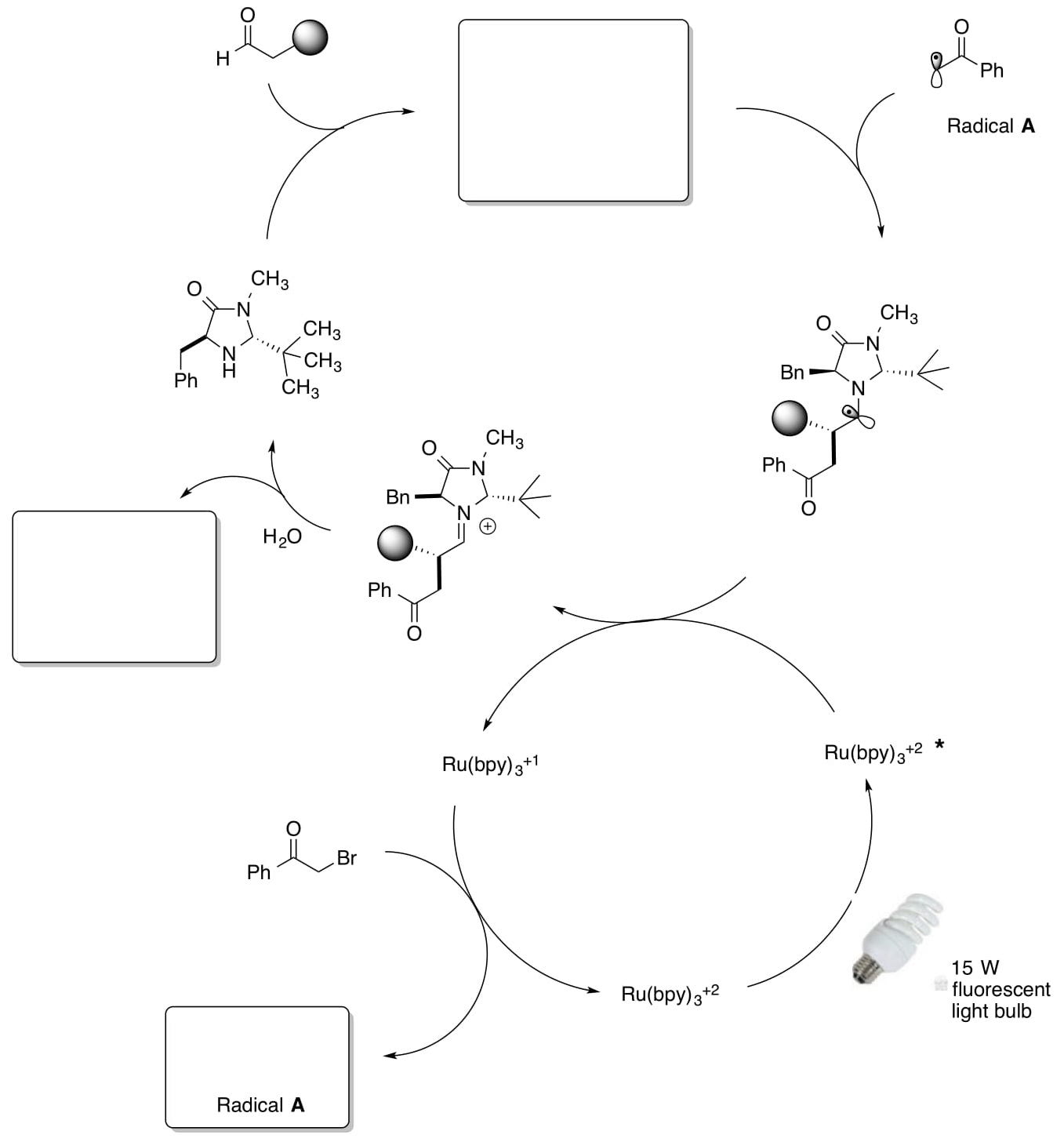
- How does the electron flow differ from the SOMO catalytic cycle?
Application Problem
- Metal-freeAsymmetricOrganophotoreduction
Neumann, Füldner, König, Zeitler Angew. Chem. Int. Ed. 2011, 50 (4): 951–954.The high cost and potential toxicity of the ruthenium salts as well as their limited availability in the future are disadvantages of these metal-based methods.
Stimulated by the attractiveness of using green light, the most abundant part of solar light, the authors speculated that a number of red to orange dyes would be a versatile metal-free alternative for the cooperative organocatalytic asymmetric a-alkylation of aldehydes.
- If you are replacing the Ru(bpy)3, what λ would you need?
- If you are replacing the Ru(bpy)3, what E° would you be trying to find?
- Comparing the photochemical properties and the reduction potentials to Ru(bpy)3, which dye would you predict to be most likely to work in catalytic photoredox? Why?
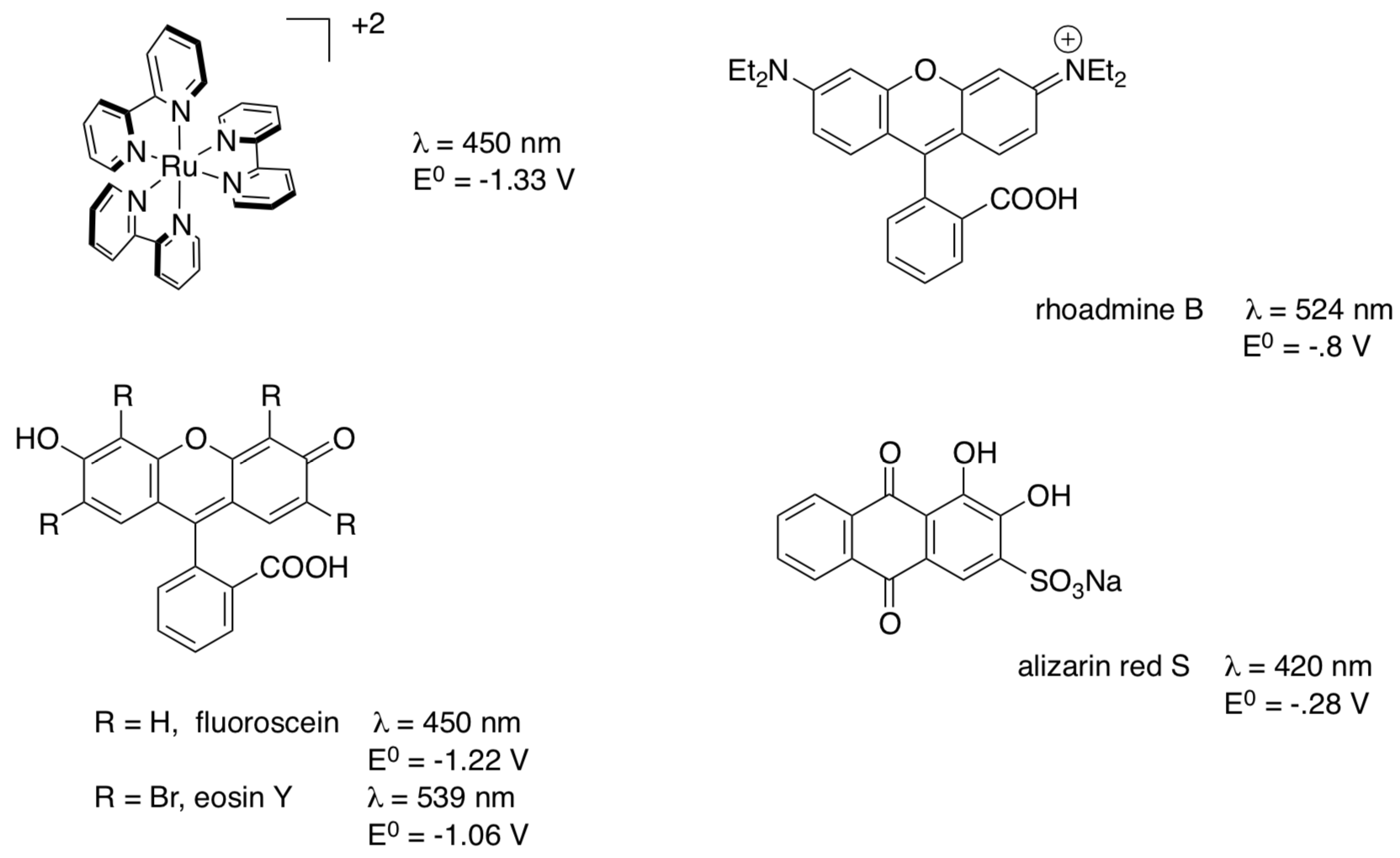
- Metal-freeAsymmetricOrganophotoreduction(cont.)
The authors were able to complete the following reaction with the MacMillan SOMO catalyst and eosin Y.
- Propose the catalytic cycle for this transformation.
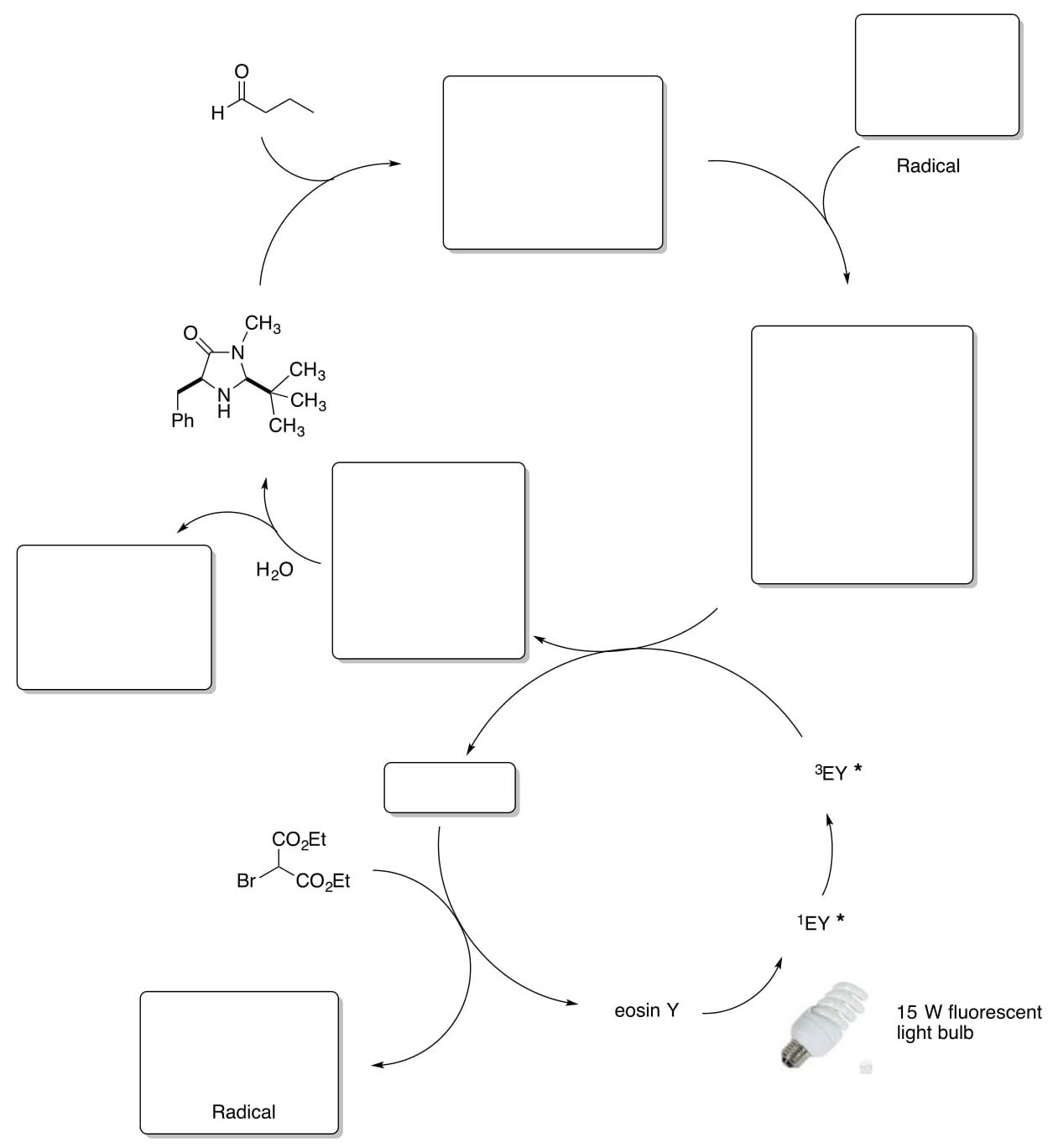
- Propose the catalytic cycle for this transformation.
- Organofluorine Compounds in Medicinal Chemistry
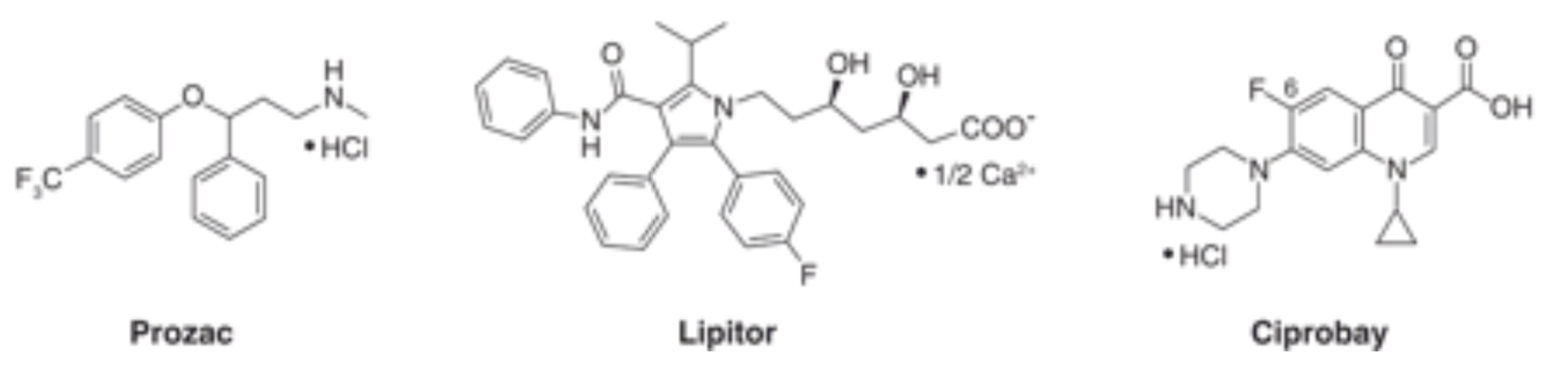
The development of methods for the construction of organofluorine compounds is of great importance due to the presence of fluorine in 20% of pharmaceuticals such as Januvia (Sitagliptin), Celebrex (Celecoxib), Prozac (Fluoxetine), and Avodart (Dutasteride).
While trifluoromethyl groups possess many desirable characteristics such as electron- withdrawing character, high lipophilicity, and excellent metabolic stability, their installation is often far from routine.
Müller et al., Science, 2007, 317 (5846): 1881-1886
- On the picture below, indicate why the presence of fluorine(s) might decrease basicity of the following pharmaceuticals. Increased lipophilicity allows the drugs to cross the blood-brain barrier.
Also, fluorine’s capacity to enhance metabolic stability (mainly by lowering the susceptibility of nearby moieties to cytochrome P450 enzymatic oxidation) has become increasingly clear recently.
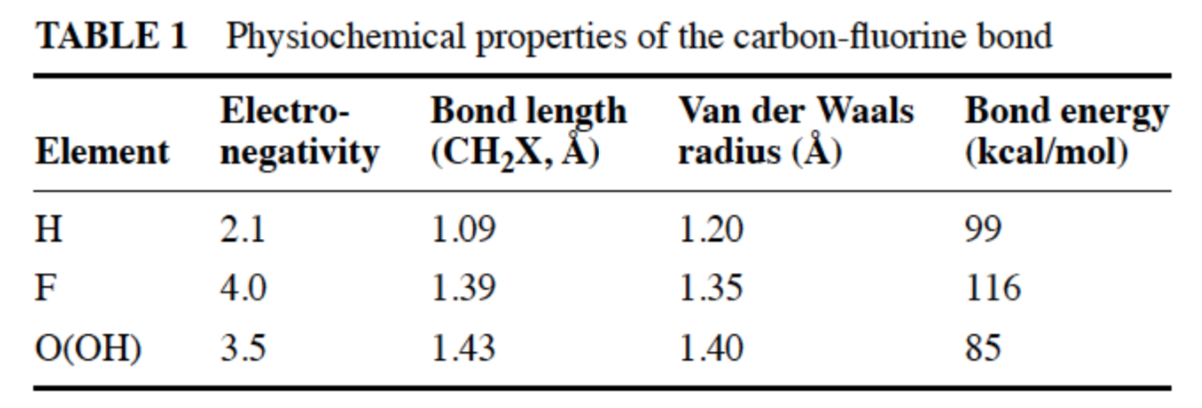
Ann. Rev. Pharmacol. Toxicol. 2001, 41, 443-70
- Examine the data in the table. Why would replacing a C-H bond with a C-F bond prevent metabolism by cytochrome P450?
- Cytochrome P450 is a major drug metabolizing enzyme (a way for our bodies to get rid of xenobiotics), why would you design a drug that could not be metabolized by cytochrome P450?
- On the picture below, indicate why the presence of fluorine(s) might decrease basicity of the following pharmaceuticals. Increased lipophilicity allows the drugs to cross the blood-brain barrier.
- Organofluorines in medicinal chemistry via Photoredox Organocatalysis (cont.)Nagib, Scott, and MacMillan, J. Am. Chem. Soc., 2009, 131 (31), 10875-10877.
Nagib & MacMillan, Nature, 2011, 480, 224-228.
MacMillan’s group has also accomplished enantioselective α-trifluoromethylation.
- Based on the previous photoredox work by MacMillan, propose a catalytic cycle for this transformation:
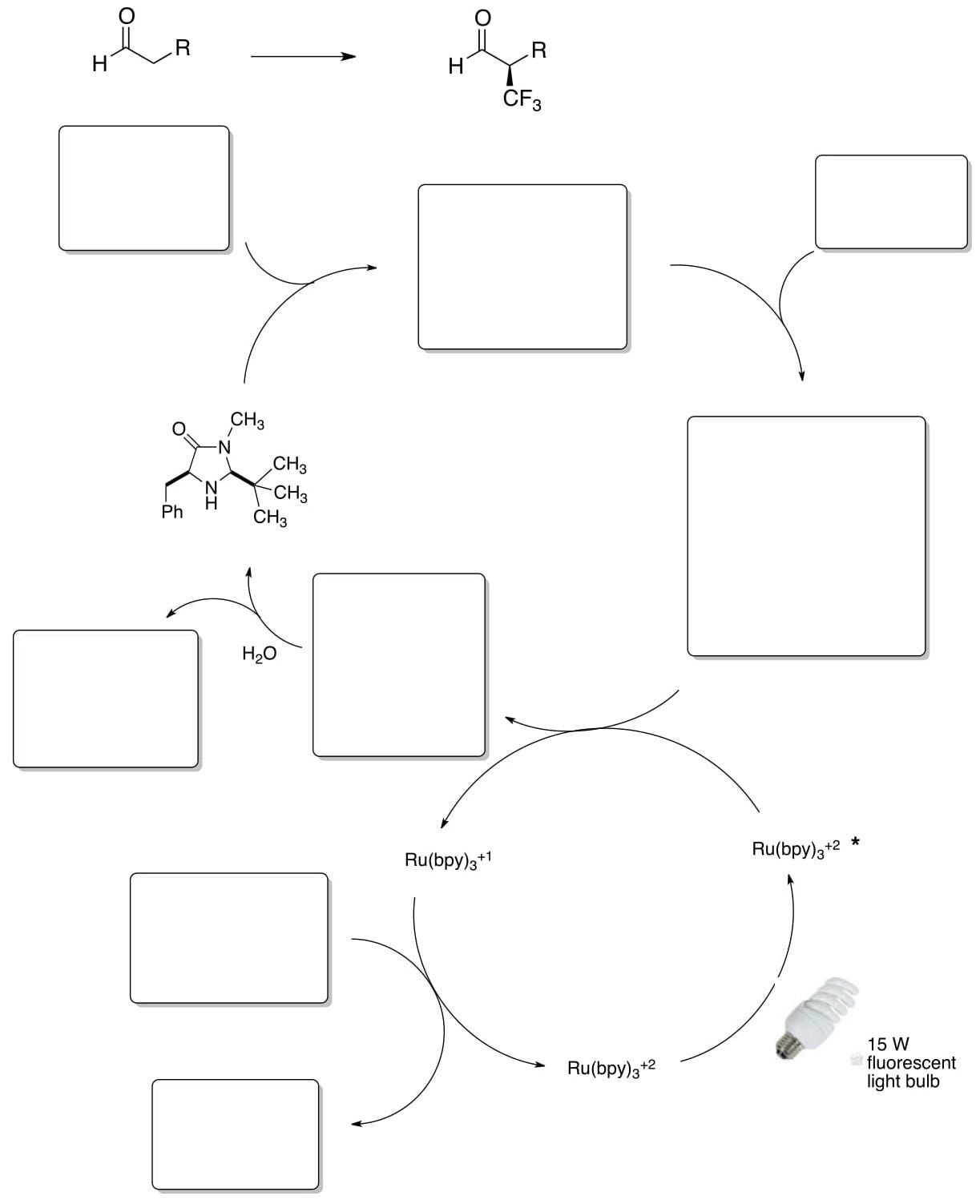
- Based on the previous photoredox work by MacMillan, propose a catalytic cycle for this transformation:
- Photoinduced RAFT polymerization
Fu, Xu, Tao and Boyer, ACS Macro. Lett. 2014, 3, 633-618.- Complete the scheme for the RAFT polymerization.
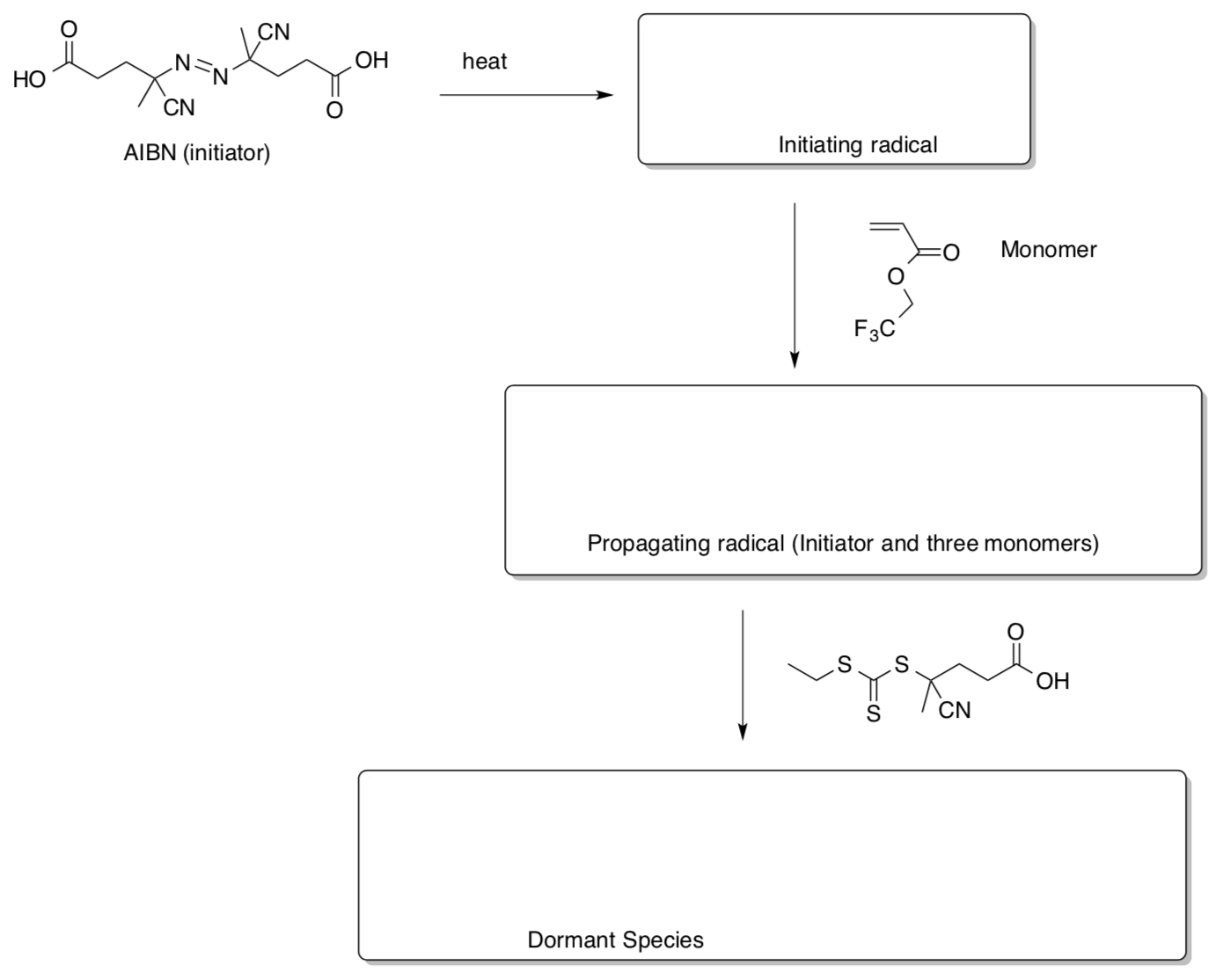
The authors were able to control the equilibrium between the dormant species and the propagating species using light.
- Show arrows for this reaction.
- Label the oxidizing species and the reducing agent.
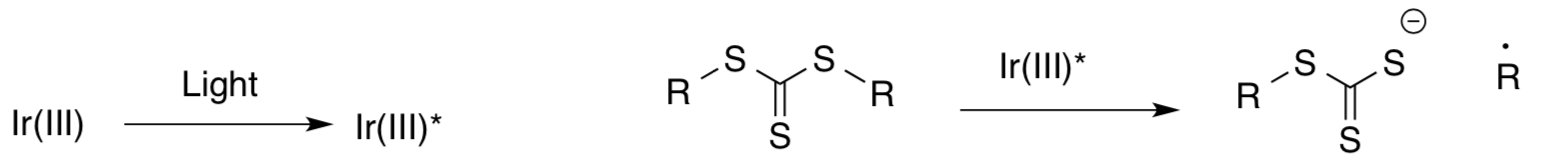
- Why is light needed to complete this reaction?
- What is the advantage of using light to regulate the equilibrium?
- Complete the scheme for the RAFT polymerization.


