21: Photochemistry of Transition Metal Complexes
- Page ID
- 150977
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Lability
Review: Inert and Labile
- Complexes that do not exchange ligands rapidly are said to be [ inert / labile ] .
- Complexes that exchange ligands rapidly are said to be [ inert / labile ] .
- Which type of octahedral complexes are more reactive to ligand exchange? Write a list of rules that will predict lability:
- size of charges:
- atomic radius:
- eg (M-L sigma*) occupancy:
- d electron configuration in octahedral complexes:
- d0
- d3
- d10
Photochemistry of Transition Metal Complexes
Photochemistry can be used to prepare compounds cleanly under mild (non-heating) conditions.
- Draw a d orbital splitting diagram for [Mo(CO)6].
- This compound is inert to thermal substitution (heating) but undergoes photochemical substitution. Explain why.
When the compound is photolyzed, the solution is also typically flushed with nitrogen during photolysis. Under these conditions, complexes with carbonyl ligands are particularly likely to undergo photochemical substitution.
- Explain why. (Hint: Consider Le Chatelier’s Principle)
It is also true that if a metal complex has multiple CO’s, the first one is lost with relative ease, the second with greater difficulty, the third with great difficulty, and any that remain at this point will stay attached.
- Explain why the loss of CO’s gets harder. (Hint—what kind of bonding does CO exhibit with a metal? What will happen to the electron density on the metal as each CO gets lost?)
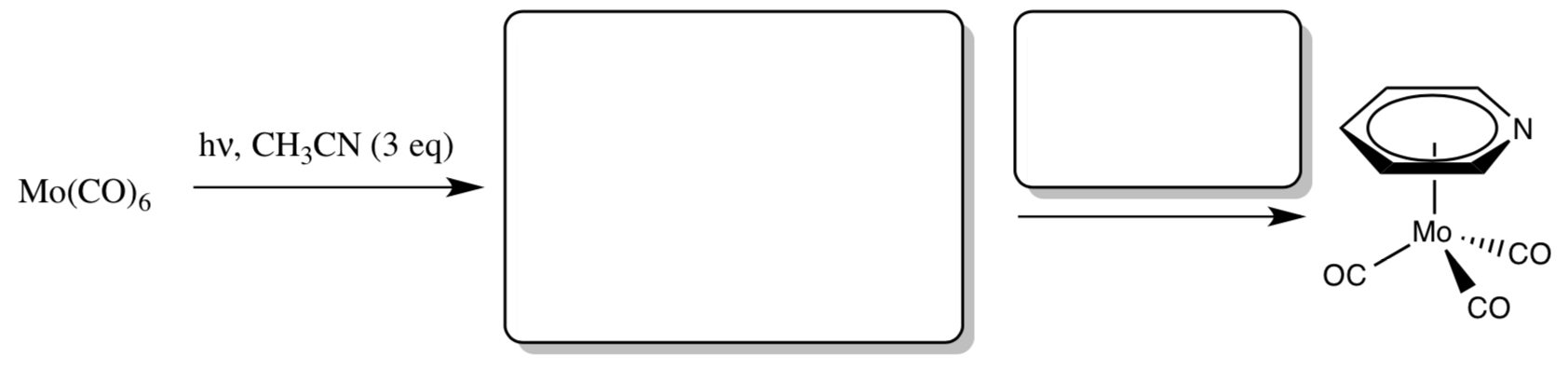
- Give a synthesis of [(η6-C6H6)Mo(CO)3] from Mo(CO)6.
Synthesis of Piano Stool Complexes
- Propose a synthesis of the following compounds from [Mo(CO)6].
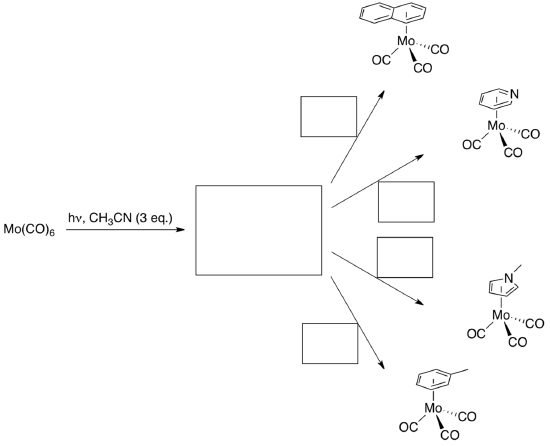
- Based on this information, how strongly bound do you think that the CH3CN ligands are?
- Explain the benefit of using [Mo(CO)3(CH3CN)3] as the intermediate species in your syntheses.
Photolysis of CO Complexes
Take a closer look at the strength of acetonitrile binding compared to CO.
- Cleavage of the M-CO bond requires:___________
- Cleavage of the M-NCCH3 bond requires:__________
Consider the pi backbonding (pi accepting ability) in these species.
- Circle which atom binds to the metal in each of these compounds:

A diatomic MO interaction diagram for a metal binding to the pi* of CO and a metal to the pi* of the CH3CN. The pi and pi* orbitals are not symmetric because the electrons are not shared equally.
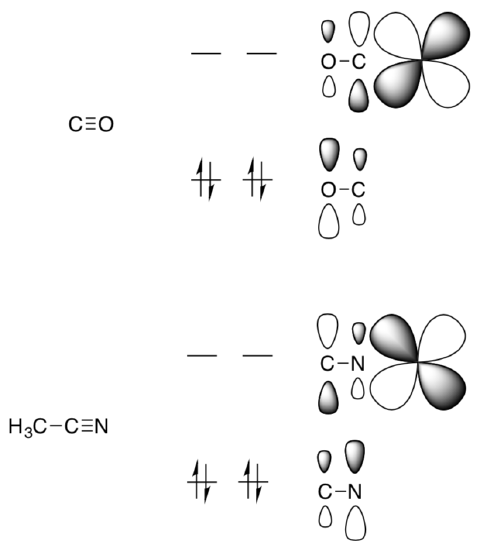
- On the diagram, indicate which direction the electrons are donated (ie from the metal to the pi* or from the pi* to the M d orbital).
- Why are the orbital lobes larger on the O/N of the pi bond?
- Considering the “backbonding” with these p* orbitals. In which case is this interaction stronger?
- Why is the CH3CN ligand more labile than CO?
Flash Photolysis: Studies of Fast Reactions
Photolysis is sometimes used to rapidly generate reactive intermediates and then monitor their subsequent reaction.
Peter Ford at UC Santa Barbara and Carmen Works at Sonoma State University reported on a flash photolysis study of a simple model compound for hydrogenase.
Other investigators had reported that hydrogenase is strongly inhibited by CO, but that inhibition is reversed with UV light. Ford and Works sought to get a better picture of this reaction.
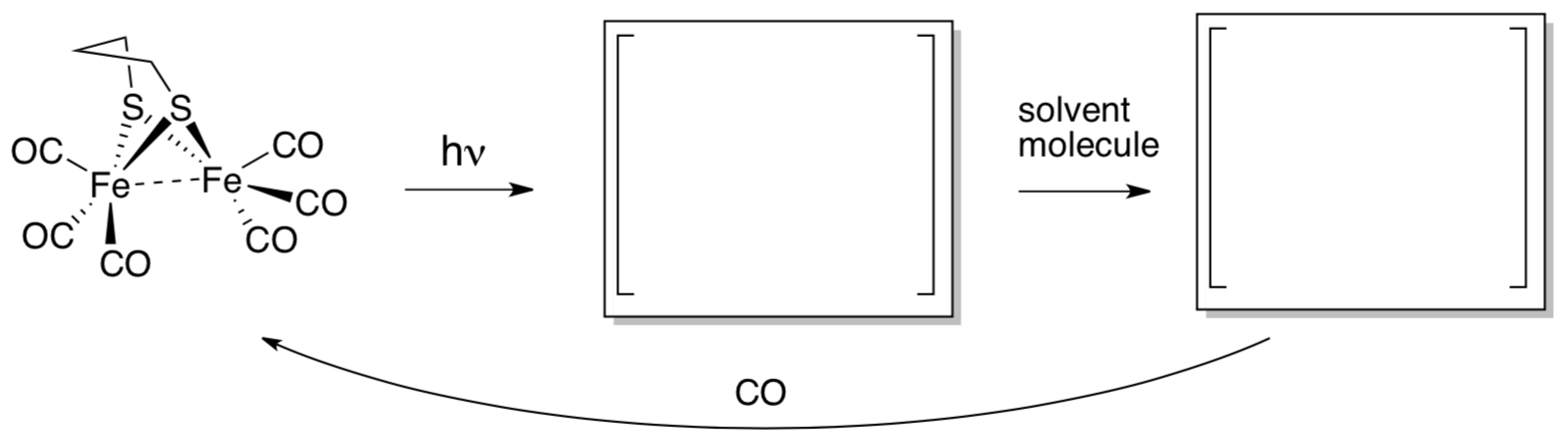
- Fill in the blanks.
- What is the electron count and oxidation state on iron?
- This low oxidation state is unusual for iron. Why is it stable here?
- What spectroscopic method might be used to monitor the formation of the “product” (starting material)?
The reaction is first order in [CO]. The following rate constants for re-formation of the original complex were measured in different solvents:
Hexanes: k = 1.0 x 108 M-1s-1
Toluene: k = 1.2 x 106 M-1s-1
THF: k = 0.90 M-1s-1
- Draw the structures of these solvents and propose a reason for the different rates.
Photochemistry of Coordination Complexes: MLCT and LMCT
If the ligand molecular orbitals are full, an electron may transfer from the ligand molecular orbitals to the empty or partially filled metal d-orbitals. This process is called ligand-to-metal charge-transfer (LMCT).
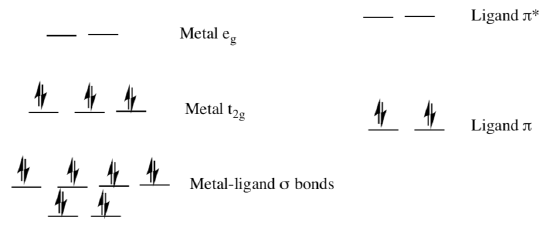
- Draw two possible LMCT transitions in the MO diagram above.
- What type of ligands would be most likely to be involved in LMCT?
σ donors | π donors | σ acceptors
- LMCT results in the [ reduction / oxidation ] of the metal.
- LMCT results in the [ reduction / oxidation ] of the ligand.
If the metal is in a low oxidation state (electron rich) and the ligand possesses low-lying empty orbitals then electrons in the metal orbitals can be excited to the ligand π* orbitals. This process is a metal-to-ligand charge transfer (MLCT).
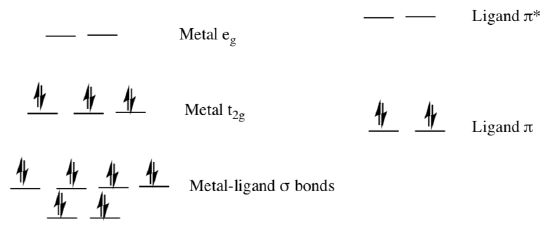
- Draw a MLCT transition in the MO diagram above.
- What type of ligands would be most likely to be involved in MLCT?
σ donors | π donors | σ acceptors
- MLCT results in the [ reduction / oxidation ] of the metal.
- MLCT results in the [ reduction / oxidation ] of the ligand.
- Why are MLCT and LMCT transitions more likely than d-d transitions?
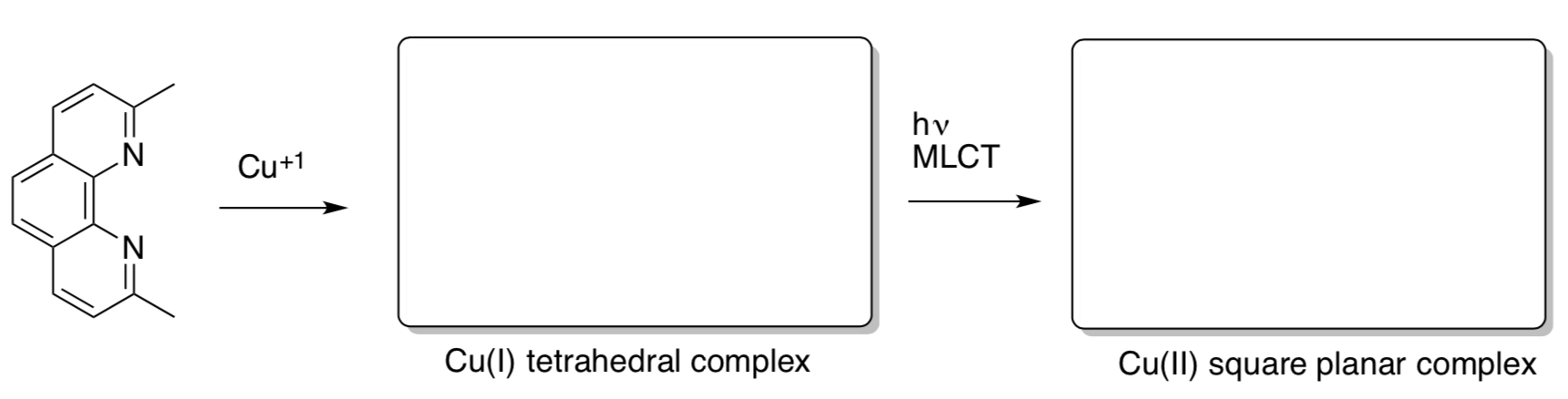
MLCT Example
The molecule 2,9-dimethyl-1,10-phenanthroline forms a complex with Cu(I) ions. Upon absorption of light, the copper ion can be changed into the +2 oxidation state through a process known as metal-ligand charge transfer.
Iwamura, M., Takeuchi, S. & Tahara, T. J. Am. Chem.Soc. 2007, 129, 5248–5256
- Complete the following reaction sequence. Include the structure of ligand with the additional electron in the square planar complex.
- What is the charge on the ligand in the square planar complex?
Summary
Piano Stool Synthesis
- Fill in the boxes with the appropriate intermediate and reagents:
- Draw cartoons of the π* orbitals of CO and CH3CN, taking the effects of atomic energy differences into account.
- Explain why photolysis can increase lability of a ligand.
MLCT and LMCT
- Define the terms:
- MLCT
- LMCT
- Why is light needed for these charge transfers?
Application Problems
- Takayoshi Suzuki at Osaka University and Jimmy Mayer at the University of Washington have investigated the photolysis of azido compounds. Metal-catalyzed processes involving azides are important in synthesis.
Inorg. Chem. 2005, 44, 8173.
The structure on the right is from an x-ray crystal structure determination.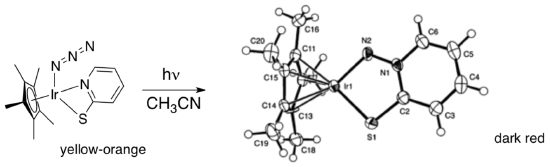
- Which bond was apparently broken? What is the side product of the reaction?
An x-ray crystal structure doesn’t distinguish between double and single bonds.
- Propose a Lewis structure for the red compound. Mark the oxidation state of iridium.
The new ligand is an example of a “redox-active” or “non-innocent” ligand, in which the ligand can participate in oxidation state changes together with the metal. These ligands can play important roles in promoting catalytic processes.
- Beside your Lewis structure, draw a second in which the oxidation state of iridium has changed by +/- 2. The charge on the free ligand will also change.
The reaction is thought to involve photolytic dissociation of the pyridine, followed by nucleophilic substitution of pyridine for dinitrogen at the azide ligand.
- Show this mechanism.
- Propose a product of the following related reaction.
- Basolo and coworkers at Northwestern University (Chicago) reported the following two reactions of pentaaminerhodium(III) azide, centre. (J. Am. Chem. Soc. 1974, 96, 1363-1369).
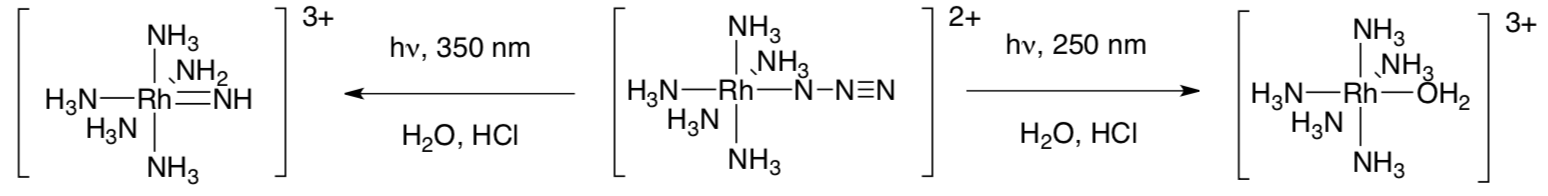
- In each case, there are other side product(s). Fill them in.
- Propose a mechanism, using curved arrows, for the reaction at 250 nm.
- Propose a mechanism, using curved arrows, for the reaction at 350 nm.
- One transition appears to involve ligand-to-metal charge transfer, whereas the other appears to be a d to d transition. Show just part of an MO diagram, with labels, and show each transition.
- LMCT
- d-d
- The photochemistry in these reactions is more complicated than the obvious mechanisms would suggest. However, given the following extinction coefficients, identify which transition belongs with which peak.
350 nm: 450 M-1 cm-1
250 nm: 8,500 M-1 cm-1
- For some transition metal complexes, we need to consider possibilities other than d to d transitions. Remember that the d orbital splitting diagram represents only the middle of the complete MO diagram. Below the d orbitals are filled bonding MO’s and, depending on the complex, additional lone pair electrons on the ligands (in the case of pi donor ligands). Above the d orbitals may be low-lying but empty π*antibonding orbitals from the ligands (if the ligand has a pi system). This will be the case if the ligand is pi accepting.
- Consider MnO4-, a tetrahedral ion that is an intense purple color. What is the d electron configuration for the metal? Can there be a d to d transition responsible for this color?
This color is due to the transition of an electron in a nonbonding lone pair on O (let’s call it a p orbital) to the metal d orbital. This is an example of what is called a LMCT (Ligand to metal charge transfer) or CTTM (charge transfer to metal) band.
- Draw a quick sketch of the relevant parts of overall MO diagram, showing the ground and excited states. Since the color is described as “intense” (ε~10,000 M-1 cm-1) explain how the transition is LaPorte and spin allowed.
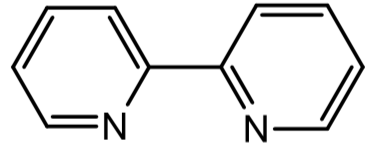
- An example of the opposite type of charge transfer band (MLCT or CTTL) occurs in [Ru(bipy)3]2+. Bipy is a bidentate neutral ligand as shown below. What is the d electron count on Ru? Would you guess it is high spin or low spin? Again, a large value of e for a peak in the spectrumallows us to assign it as a charge transfer band. Since there are d electrons in this case, and there are empty p* orbitals on the ligands, this transition has been assigned as MLCT or CTTL. Draw a “complete” (generic) MO diagram to show the transition.