20: Introduction to Photochem
- Page ID
- 150645
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Introduction to Photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of the absorption of ultraviolet (wavelength from 100 to 400 nm), visible light (400 – 750 nm) or infrared radiation (750 – 2500 nm).
Electronic Excitation
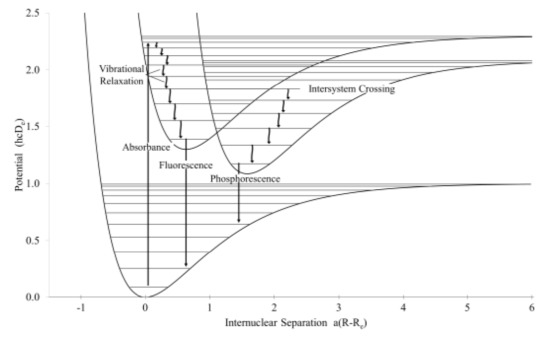
One way to describe the possibilities is to use a diagram that shows the relative energies of excited states as a function of inter-nuclear distance.
- Label the axes on the diagram shown below.
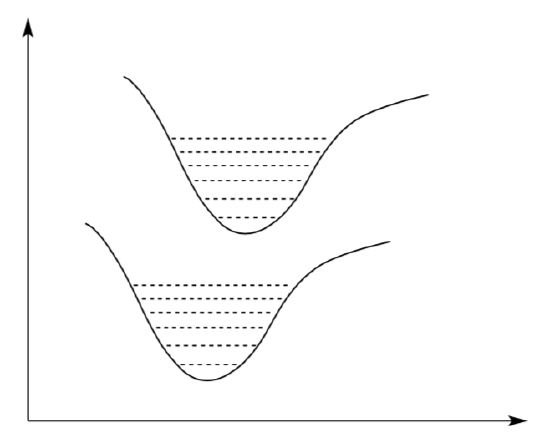
The horizontal lines represent vibrational states for each of the electronic states.
- Draw an arrow on the diagram to show the transition associated with the molecule absorbing infrared light (i.e. a bond vibration).
The two wells indicate orbital levels.
- Draw a line on the diagram to show an electronic transition between the ground state and an excited state.
In the diagram above, the “bottom” of the potential well in the excited state is displaced toward longer bond lengths compared to the ground state.
- Complete the MO diagram after excitation of an electron and explain why the bond length increases.
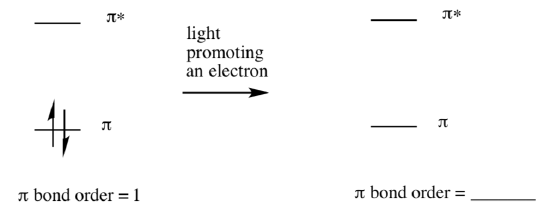
Franck-Condon Principle
The Franck-Condon Principle states that transitions between electronic states correspond to vertical lines in the diagram.
- Which weighs more?
Electrons OR Nuclei - Which is easier to move?
Elections OR Nuclei
- Which would be faster?
electronic transitions OR bond length changes
Thus, during light absorption, (femtoseconds), electrons can move, not the nuclei.
The much heavier atomic nuclei have no time to readjust themselves during the absorption but can readjust afterward the absorption.
- The readjustment is accomplished with ________________.
Different geometries for the ground state and the excited state can affect the ability of the molecule to undergo an electronic transition.
- Which of these two electronic transitions is more likely? Explain.
Chromophores and Selection RulesWhich electrons can be excited? Where do they go?
Obtaining a uv-visible spectrum can provide other useful information about the compound being studied. A chromophore is the part of a molecule that absorbs uv light.
An MO diagram for formaldehyde (methanol, CH2O) is shown.
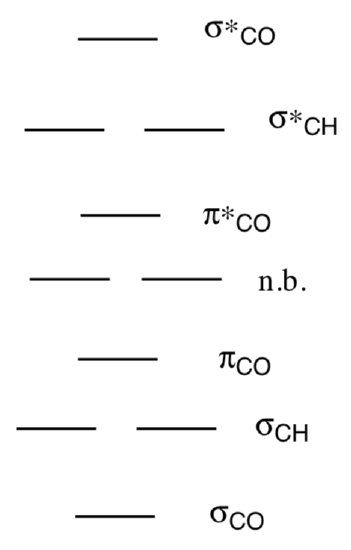
- Place the proper number of electrons in the diagram.
There are 4 common types of transitions (n-π*, π-π*, σ-σ*, n-σ*) for organic compounds
- Molecules with chromophores tend to have a lot of pi bonds. Explain why.
Extinction Coefficients
Beer’s law states that the absorbance of a solution is proportional to its concentration. Mathematically, Beer’s law is
A = εbc
A is the absorbance of the solution
ε is a quantity called the molar absorptivity
b is the path length in cm of the solution cell
c is the solution concentration (molarity)
Absorbance spectra are usually gathered as a function of wavelength. Absorbance is dependent upon concentration.
- Suppose that a uv absorbance spectrum was obtained for a solution of a compound. Then the concentration of the solution was doubled. Redraw the new spectrum on the same scale.
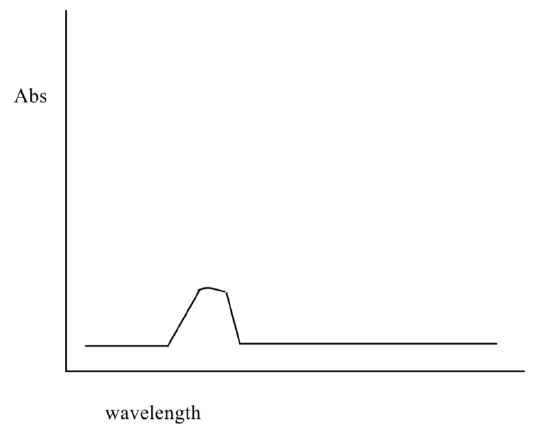
Absorbance is also dependent the molar absorptivity. Molar absorptivity (ε) tells you how likely an electronic transition is.
- The more likely a particular absorbance occurs, then the [ smaller / larger ] the ε will be.
- If an electronic transition is difficult, _______ [ more / fewer ] electrons will be excited, and the ε will ________ [ decrease / increase ] and the apparent intensity will be weaker.
- σ->σ* has a small extinction coefficient. Why?
Selection Rules
The following two selection rules predict whether an electron is likely to undergo a specific electronic transition.
A “forbidden” transition doesn’t mean that it CANNOT happen, just that it has a lower probability of occurring.
- Transitions between orbital of like symmetry are forbidden. (This is called the LaPorte selection rule.)
- A transition between two orbitals of the same type (e.g. d to d) is forbidden.
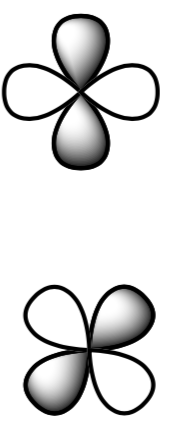
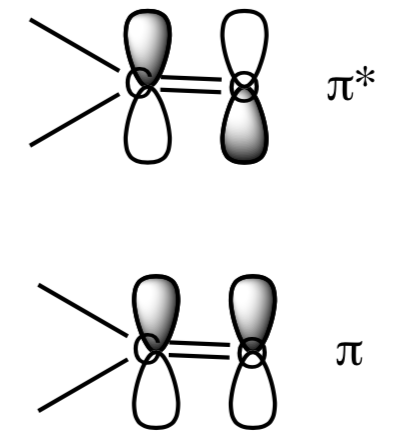
- Transitions between a pi orbital and a pi* orbital are allowed. The pi and pi* orbitals of a ketone are shown. What is different about their symmetry?
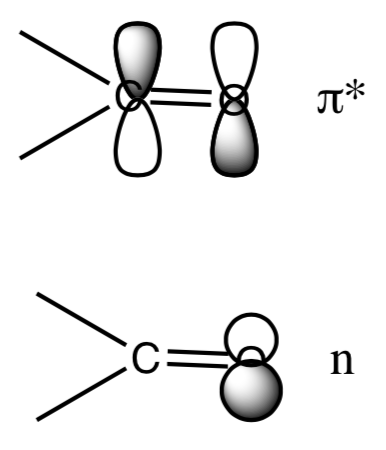
- n->π* are the lowest energy of any transitions. However, they typically have the smallest extinction coefficient. Why is the n->π* transition so difficult even though the energy gap is small? Consider how much orbital overlap is present.
- A transition between two orbitals of the same type (e.g. d to d) is forbidden.
- Transitions involving change in net electron spin are forbidden.
In a triplet state, there are two unpaired electrons with parallel spins.
In a singlet, the electrons are either paired or they are in different orbitals but with antiparallel spins.
- Draw the d orbital diagram for a d1 metal ion in an octahedral field. How many unpaired electrons are there?
- Redraw the diagram after the system has absorbed light. This is called an “excited state”. Now how many unpaired electrons are there? This is called a “spin allowed” transition.
- In an octahedral field, draw the following:

- Now draw a spin allowed transition for each. [Hint—if electrons are paired within an orbital and then the spins remain opposite in an excited state they count as “paired” (even when they are no longer within the same orbital.)
- On the MO diagram for formaldehyde and draw a spin-allowed transitions.
Fluorescence, Phosphorescence and Photosensitization
Fluorescence
Suppose an electron is excited but it quickly drops back down to where it came from. If it did, it might give off a photon. This process is called “resonance fluorescence.”
- Compare the wavelength of light that would be emitted to the wavelength originally absorbed by the electron.
- Would this transition be allowed or forbidden?
- Suppose a geometric change occurs (such as a bond length changing). How will that affect the ability to undergo resonance fluorescence?
Resonance fluorescence is not very common and it is more likely to happen in the gas phase than in solution.
Generally, collisions between molecules in solution cause the molecule to lose energy and drop to the ground vibrational state of S1. Then the electron can return to the ground state. Fluorescence is more likely to occur this way.
- In this case, the energy of the photon emitted is [ greater than / less than ] the energy of the photon absorbed.
In fluorescence, transitions are between two singlet states.
- Is the transition spin-allowed?
- Knowing about the regular mode of fluorescence, explain why resonance fluorescence is more likely to occur in the gas phase than in solution.
If it is allowed, it is probable, and the lifetime of an excited state is typically on the only order of 10-7 second in fluorescence.
Phosphorescence
Supposed you were asked to draw the ground state configuration for the valence electrons in the p orbitals on a nitrogen atom. Three possibilities are shown below.
- Circle the correct choice:
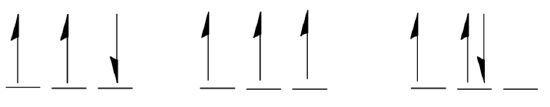
Hund’s rule allows us to pick the ground state. Now label the multiplicity of each of the three possibilities (recall multiplicity = (number of unpaired electrons +1) , and electrons with opposite spins but in different orbitals are considered spin-paired.)
Note that the higher spin state is actually lower in energy.
- Sketch a singlet excited state using the MO diagram for formaldehyde.
- Show the triplet state for formaldehyde.
- Which should be lower in energy?
Depending on the electronic state of a molecule, a singlet excited state can convert to a lower energy triplet state. (This is called intersystem crossing.) In order to return to the ground state, the electron spin must flip again. This is a low-probability event and so the lifetime of the excited state is long. In phosphorescence, the excited state lifetime is 10-3 second or longer.
Wikipedia (“phosphorescence”; accessed November 30, 2012)
Radiationless Relaxation
An electron can also relax without giving off a photon.
Vibrational Quenching
- Explain how collisions with other molecules might allow the molecule to return to the ground state.
- Vibrational quenching is more common in polar solvents than non-polar solvents. Explain why.
- How will the time frame of vibrational quenching compare to fluorescence?
Photosensitization
- What happens when a moving billiard ball strikes a stationary billiard ball sitting on the table?
- Why does that happen?
In photosensitization, a molecule in a triplet excited state collides with another molecule and transfers its extra energy.
- How will the time frame of photosensitization compare to fluorescence?
Photosensitization can be an important pathway for energizing a molecule that lacks a proper chromophore.
- Explain why.
Summary:
- Infra-Red Spectroscopy involves absorption of IR energy resulting in:
- UV-Vis Spectroscopy involves absorption of UV-Vis energy resulting in:
- What are the two Selection Rules?
- Laporte (symmetry):
- Spin:
Transition ε (M-1 cm-1) Example Fully spin and Laporte allowed 3,000-10,000 and above Metal to ligand charge transfer or
π to π* (alkenes or aromatic systems)Spin allowed, Laporte forbidden 100-400 Transition metal d to d, complex tetrahedral Spin allowed, Laporte forbidden 20-150 n-π*, organic molecule Spin allowed, Laporte forbidden 5-50 Transition metal d to d, complex octahedral Spin forbidden, Laporte forbidden 0.1-1 Transition metal d to d (oct or tet) with change in spin multiplicity
- Define the following terms:
- Molar Absorptivity:
- Singlet vs Triplet States
- Phosphorescence:
- Fluorescence:
- Photosensitization:
Applications:
- Sensor for Singlet Oxygen
Singlet oxygen is highly reactive especially in biological systems. Singlet oxygen will oxidize intracellular organelles and can lead to cell death. For these reasons, there has been much demand to develop a detection method.
Kim, Fujisuka, & Majima, J. Phys. Chem. B, 2013, 117, 13985-13992.
Singlet Oxygen Sensor Green
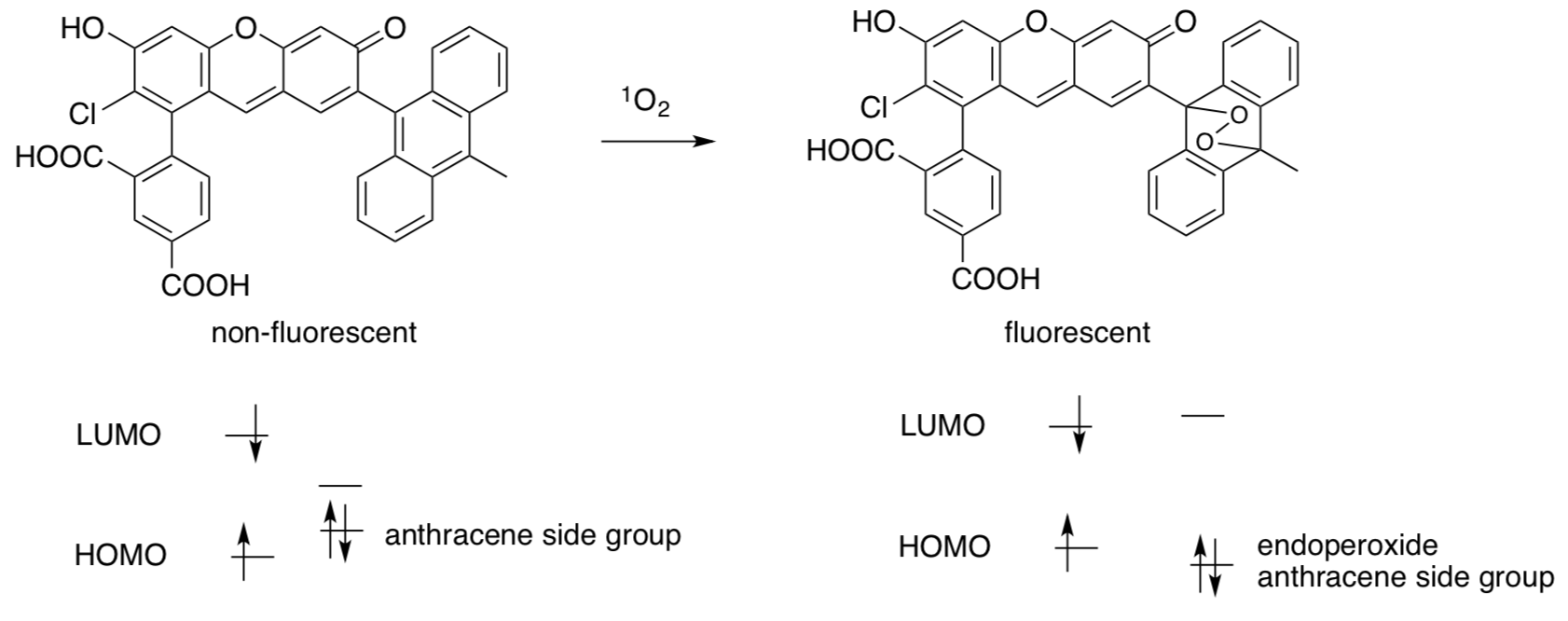
After absorption of light, the sensor (shown on left) is non-fluorescent because an electron is transferred to the HOMO level too quickly to observe any fluorescence.
- In the diagram above (left), draw the electron transfer responsible for the quenching (prevention of fluorescence) in the non-fluorescent structure.
- Complete the Lewis structure after this electron has been transferred.
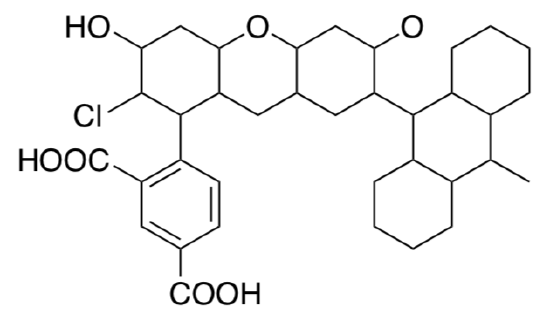
- After the sensor reacts with singlet oxygen, the Singlet Oxygen Sensor fluoresces green. Show what activity is responsible for the emission of light on the MO diagram above (right).
- Why doesn’t the anthracene side chain quench the fluorescence in the structure on the right (after reaction with singlet oxygen)?
- Many “glow in the dark” toys are phosphorescent--for example, plastic stars that glow on the ceiling when room lights are turned off. Now, explain how they work.
- “Light sticks” are not phosphorescent but chemiluminescent. In this case there is a chemical reaction occurring (a relatively slow one) that produces products that are formed in an electronically excited state. They transfer energy to a dye molecule, called a sensitizer, that releases energy in the form of light via fluorescence. The release of light is related to the kinetics of the reaction. How can you get a glow stick to last longer? How can you get a glow stick to be brighter?
- Consider the following transition metal ions.
- Ti+4. (Assume octahedral geometry.) What’s the d electron count? TiO2 is the main constituent in white paint. Why? Similarly, what color are Na+ and Ca2+ salts with common anions?
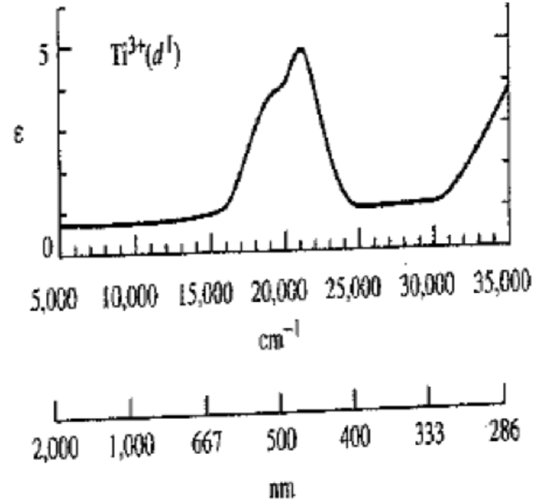
- Ti[( H2O)6]3+, d1. (See below.) Now note the value of the y axis at the wavelength of maximum absorbance. Is this consistent with our earlier explanation for the source of the color? Most other octahedral transition metal ions have spectra similar to this, although they may have more than one peak.
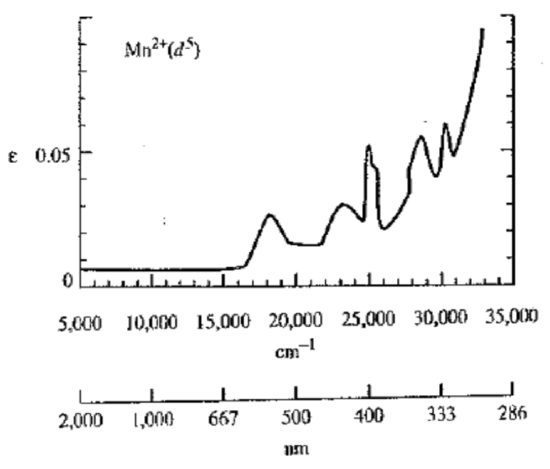
- [Mn(H2O)6]2+ (high spin). Draw the ground and excited states. What’s going on here? Note the y scale on the absorbance spectrum.
- Zn2+. No spectrum shown. Draw the ground state. What color would you expect Zn2+ to be? (Should it matter whether it is octahedral or tetrahedral or what the ligands are?)
- It is possible to prepare cobalt (II) analogues of many enzymes that contain Zn (II). The spectrum of Co(II) carbonic anhydrase (normally a Zn(II)-containing enzyme) showed peaks at the following positions: 520 nm (ε=205 M-1 cm-1); 555 nm (ε=340 M-1 cm-1); 615 nm (ε = 230 M-1 cm-1); 640 (ε=240 M-1 cm-1).
- What is the geometry (coordination number and arrangement of ligands) around the metal in the enzyme? Explain how you know.
- Why didn’t the scientists just analyze the “native” (Zn-containing) enzyme to determine the geometry in this way?
- Window glass, when viewed from the edge, is green. This is due to the presence of small amounts of Fe2+. (Oxide ions from the silica serve as ligands for the metal.) However, colorless glass can be made by adding Mn3+. This causes a transfer of an electron from the Fe to the Mn.
- In terms of Beer’s law, why is the color more apparent when the glass is viewed from the edge rather than straight on?
- In glass, would Fe2+ likely be high spin or low spin?
- Explain why the Fe would become “colorless” upon reaction with Mn3+.
- Why wouldn’t the Mn just make the glass be a different color?
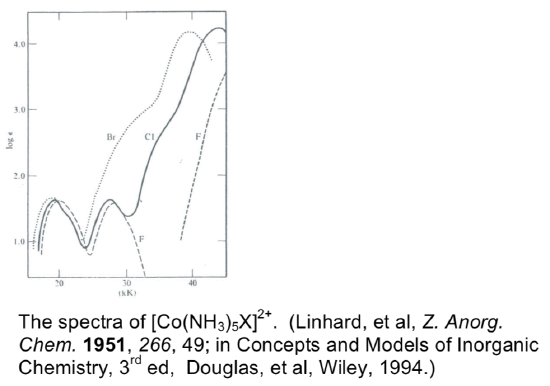
- The spectra for a series of [Co(NH3)5X]2+ complexes are shown below. Note that the y axis is a “log” scale (for example, “2” is really 102), and the units of the x axis are in 1000’s of cm-1.
- Name the complex (for X=Cl) and determine its d electron count. Is it likely to be high spin or low spin?
- Assign the transitions for the X=Cl complex. (What sort of processes are causing each of the three peaks, and how do you know?) Draw the key parts of the MO diagram and label the orbitals.
- The peak at highest energy for X=Br is shifted to higher/lower energy (circle one), compared to the Cl-containing complex. Explain this.
- It is often important to be able to quantify the amount of protein in a solution. One method is based on the fact that aromatic amino acids tyrosine (Tyr) and tryptophan (Trp) absorb ultraviolet light maximally at ~280 nm. The amino acid cysteine does not absorb at this wavelength; however, two cysteines may react with each other to form a disulfide (S-S) linkage which is called cystine and this structural feature does. The general method is to just take a solution of unknown protein sample, stick it into a spectrophotometer, and read the A280.
Based on a sample of measured molar extinction coefficient (ε) values for 80 proteins, the ε at 280 nm of a folded protein in water, ε(280), can best be predicted with this equation:
ε (@ 280) (M-1 cm-1) = (#Trp) (5,500) + (#Tyr) (1,490) + (#cystine) (125)
Of course, it is necessary to know the structure of the protein so that the number of each type of amino acid residue can be entered into the equation.
- Examine the equation and predict the type of absorption that each of the three amino acids in the equation undergoes at 280 nm.
- Draw the side chains of these three amino acids.
- Does this match your prediction? Explain.


