18f: ATP Synthase
- Page ID
- 150641
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCComplex V: ATP Synthase
Neglecting Complex II, the overall reaction of the mitochondrial chain, per 2e- transferred, can be written as:
NADH + H+ + 1⁄2 O2 + 10 H+("in") -> NAD+ + H2O + 10 H+("out") E° = +1.135V
Each two e– (from 1 NADH molecule) through the electron transport chain results in the net transfer of 10 protons across the membrane:
Complex I: ________ H+
Complex III: ________ H+
Complex IV: ________ H+
Protons will diffuse from an area of high proton concentration to an area of lower proton concentration. Peter Mitchell received the Nobel Prize in 1978 for his proposal that an electrochemical concentration gradient of protons across a membrane could be harnessed to make ATP. The proton gradient created by the electron transport chain provides enough energy to synthesize about 2.5 molecules of ATP through a process called chemiosmosis.
- This proton flow is driven by two forces (fill in the blanks):
- Diffusion force caused by a concentration gradient. All particles tend to move from __________ concentration to __________ concentration.
- Electrostatic force caused by an electrical potential gradient. An electrical gradient is a consequence of charge separation. Protons will be attracted to ___________.
ATP synthase is an important enzyme that utilizes the proton gradient drive the synthesis of (ATP).
Electric Potential Drives Motor
Dimroth, Operation of the F0 motor of the ATP synthase, Biochimica et Biophysica Acta (BBA) -Bioenergetics, 2000, 1458, 374-386.
The rotor is not locked in a fixed position in the center of the bilayer and the rotor sites switch between the empty and the ion bound states. When driving ATP synthesis, an ion arrives from the periplasm and binds at an empty rotor site.
- What is the charge of the empty binding sites a-c
- when no ion is bound?
- when a Na+/H+ ion is binding?
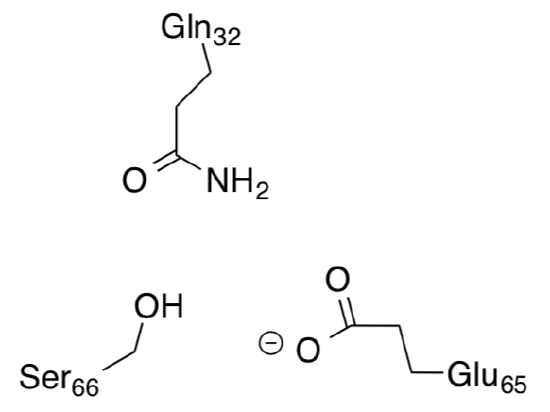
The positive stator charge (Arg227) plays a fundamental role in the function of the F0 motor.
- Draw this side chain with its charge.
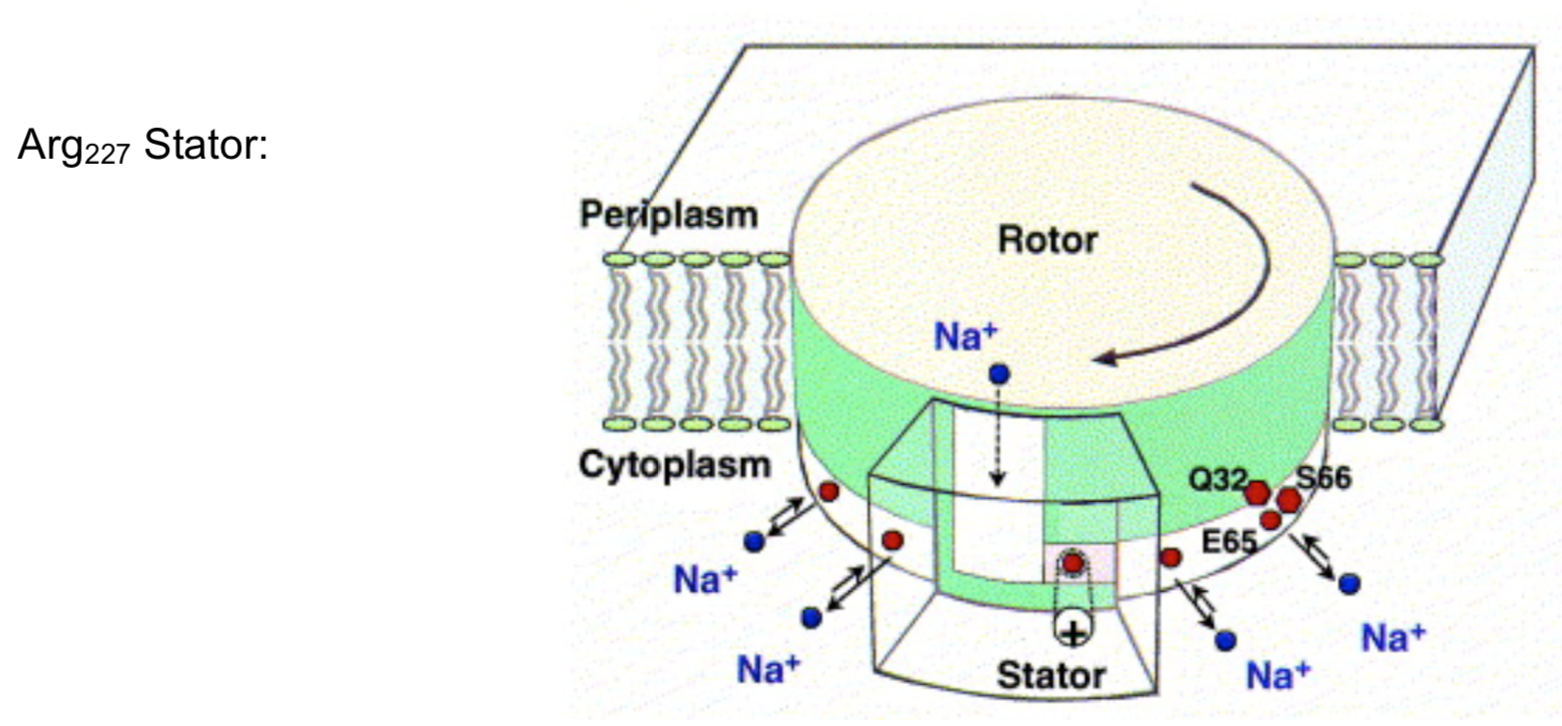
- When the rotor site has picked up an ion from the stator channel, its net charge reducing is reduced to a dipole, thus _____________ (increasing/decreasing) the attraction to the stator. The rotor moves onward through the hydrophobic part of the stator, while the arginine attracts the next empty rotor site.
- The empty site (charge = ________) is electrostatically attracted by the stator (charge = ________) and guided into the a-c interface.
- The rotor site is occupied until it reaches the stator from the opposite side, where it encounters the positive stator charge, causing dissociation of the ion. Why? Consider diffusion gradients and charges.
Electrical Power Fuels Rotary ATP Synthase
- Fill in the blanks on the following summary of ATP Synthase:
During ATP synthesis, the ____________ gradient fuels the membrane-embedded F0 motor to rotate the central stalk. This rotation causes sequential binding changes at the peripheral F1 domain so that one catalytic site binds ________ and phosphate, the second makes tightly bound ATP, and the third step ____________.
In anaerobically growing bacteria, when the respiratory enzymes are not active, the F1 motor can hydrolyze ATP.
- Which direction will the pump turn in these conditions?
- What will happen to the F0 motor? And the H+ gradient?
Ion Path
Dimroth, von Ballmoos, Meier, Kaim, Structure, 2003, 11, 1469-1473.
The path of Na+/H+ during ATP synthesis is from the periplasm through the subunit achannel onto an empty binding site on subunit c at the rotor-stator interface.
- Draw a Na+ or H+ ion into the binding site on subunit a (shown below).
After turning the rotor site out of the interface with the stator, the ion can freely exchange through its rotor channel with the cytoplasmic reservoir.
Meiers reacted the carboxylate side chain of the Glu65 in the models of the F0 motor with dicyclohexylcarbodiimide (DCC).
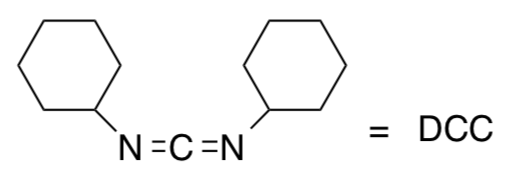
- Show this reaction.
This modification inhibited ion translocation through F0 in subunit a in the proton motor.
- What did this experiment help Meiers to predict about the role of Glu65?


