18d: Complex IV
- Page ID
- 150637
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCComplex IV: Cytochrome C oxidase (CCOx)
Complex 4 Overview
Another complex whose goal is to move electrons and protons! This is the big step since it is the main site for dioxygen utilization in all anaerobic organisms.
The structure of complex IV is shown in the left figure and to the right in a diagram taken from the Kegg pathways (with permission).
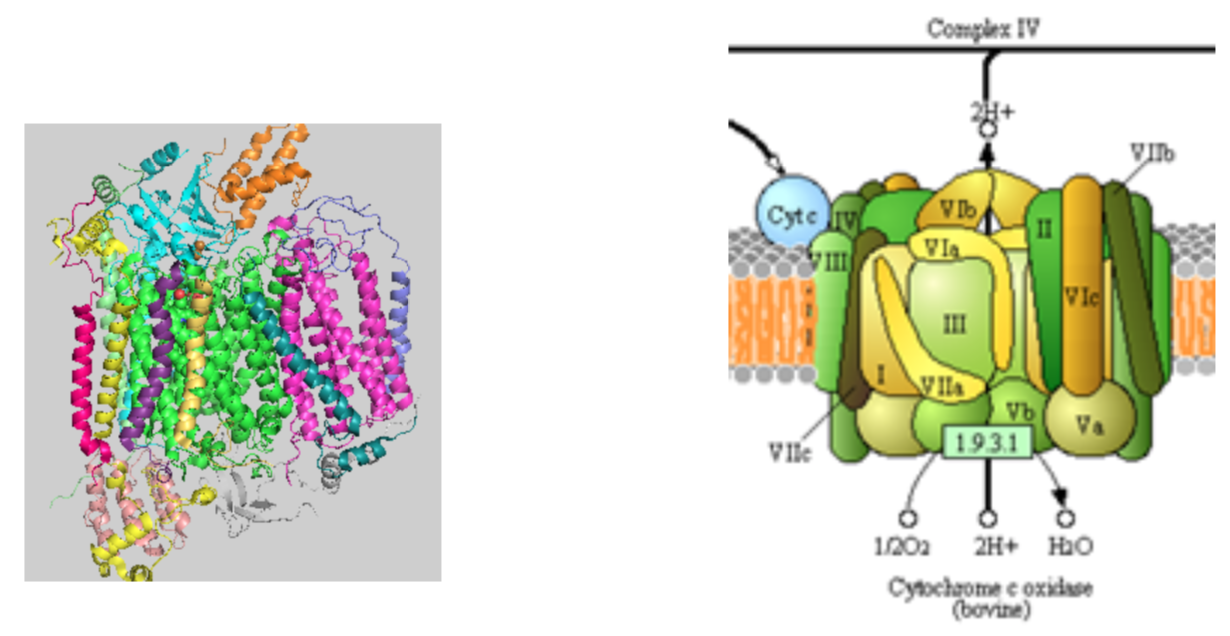
- Complete the net equation for the redox reactions of complex IV.
___cytc+2 +1O2 + 8H+ -> ___H2O + 4H+ + ___cytc+3
- Calculate the ΔG° for the net reaction of Complex III. Show your work.
ΔG° =
- How many protons are being “pumped” into the intermembrane space? _________
- How many electrons are needed to balance this equation? _________
- What are the initial and final “mobile” carriers of electrons?
- On the right diagram draw large arrows representing the directions of electron transfer and proton transfer.
- Draw the inner membrane on the figure to the left. What is the predominant secondary structure in membrane domain of the complex?
- Why is this enzyme complex called an oxidase and not a mono- or dioxygenase or a dehydrogenase?
Hemes in Electron Transport
Cytochrome c, the initial “substrate” of this complex, delivers electrons from its heme cofactor to a dinuclear copper cluster, CuA. From there, electrons flow to heme a (low spin) which transfers them to another heme a3 (high spin) and then finally to dioxygen which is coordinated to the Fe in heme a3and to an adjacent CuB.
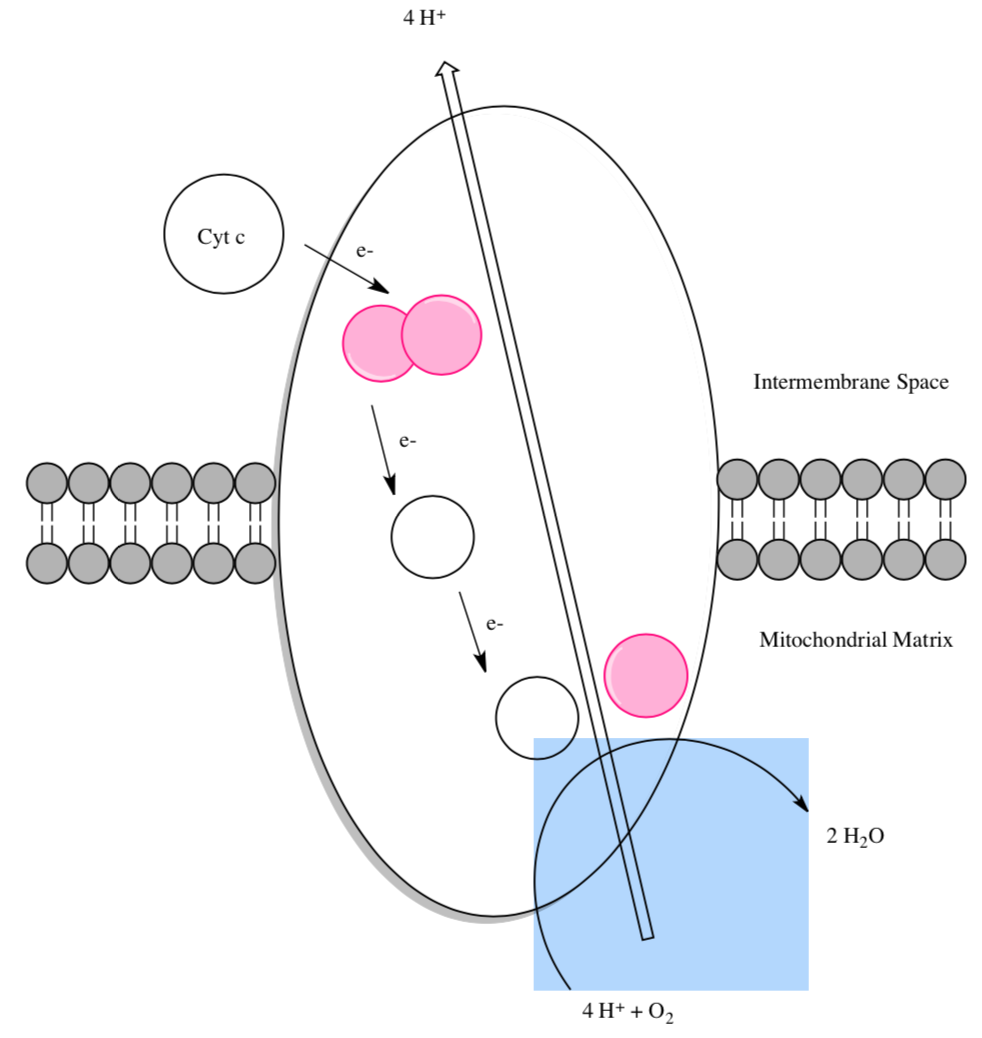
- Label all the prosthetic groups in the cartoon showing the electron flow.
- How to reduce O2, you need to add 4e-. How many electrons could be held by the metallic centers (CuA, heme a, heme a3, CuB) inside Complex IV?
There is a huge difference in mass between the electron and the proton.
- The _____________ is nearly 2,000 times heavier.
- The _____________ is much more likely to behave like wave.
Due to this difference, electrons have the ability to tunnel through long distances of space. Recent calculations seem to indicate that the transfer from CuA to heme a is due to “tunneling” (The distances observed for intramolecular electron transfer
are almost universally less than 15 Å.)
Kaila, Johansson, Sundholm and Wikström, Proc Natl Acad Sci, 2010, 107(50): 21470–21475.
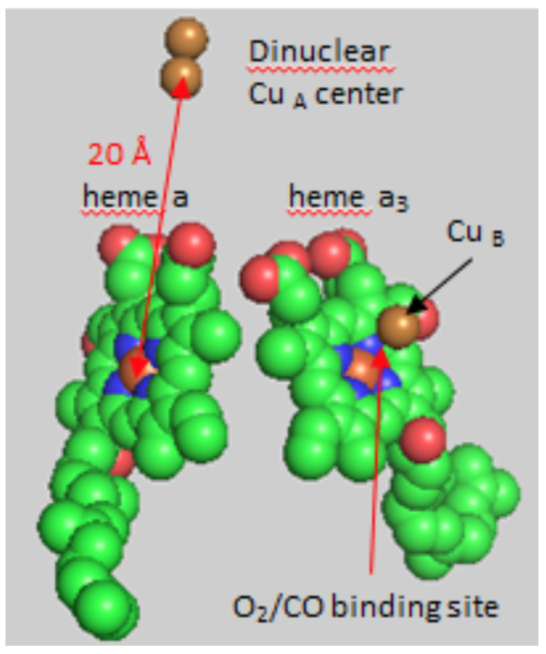
- Restate in your own words how an electron might be “transported” the 20 Å distance(!) from the dinuclear CuA cluster to the Fe in heme a and ultimately on to dioxygen.
O2 Redox Mechanism
Heme a and a3 vary from the heme in hemoglobin as they both have a formyl group.
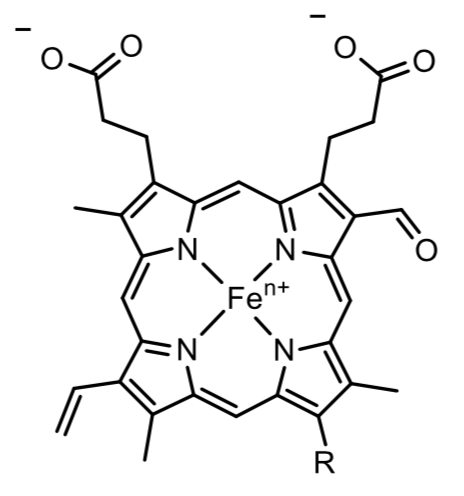
- Put a box around the formyl group.
- What is its overall charge of the heme in its reduced state?
_______ In its oxidized state? ________
- How does the formyl group affect the ability to accept e-?
The exact mechanism of O2 reduction and cleavage is not wholly understood. One proposed mechanism for the catalytic cycle of O2 reduction is shown below.
- Draw arrows for the O2 reduction catalytic cycle in Complex IV.
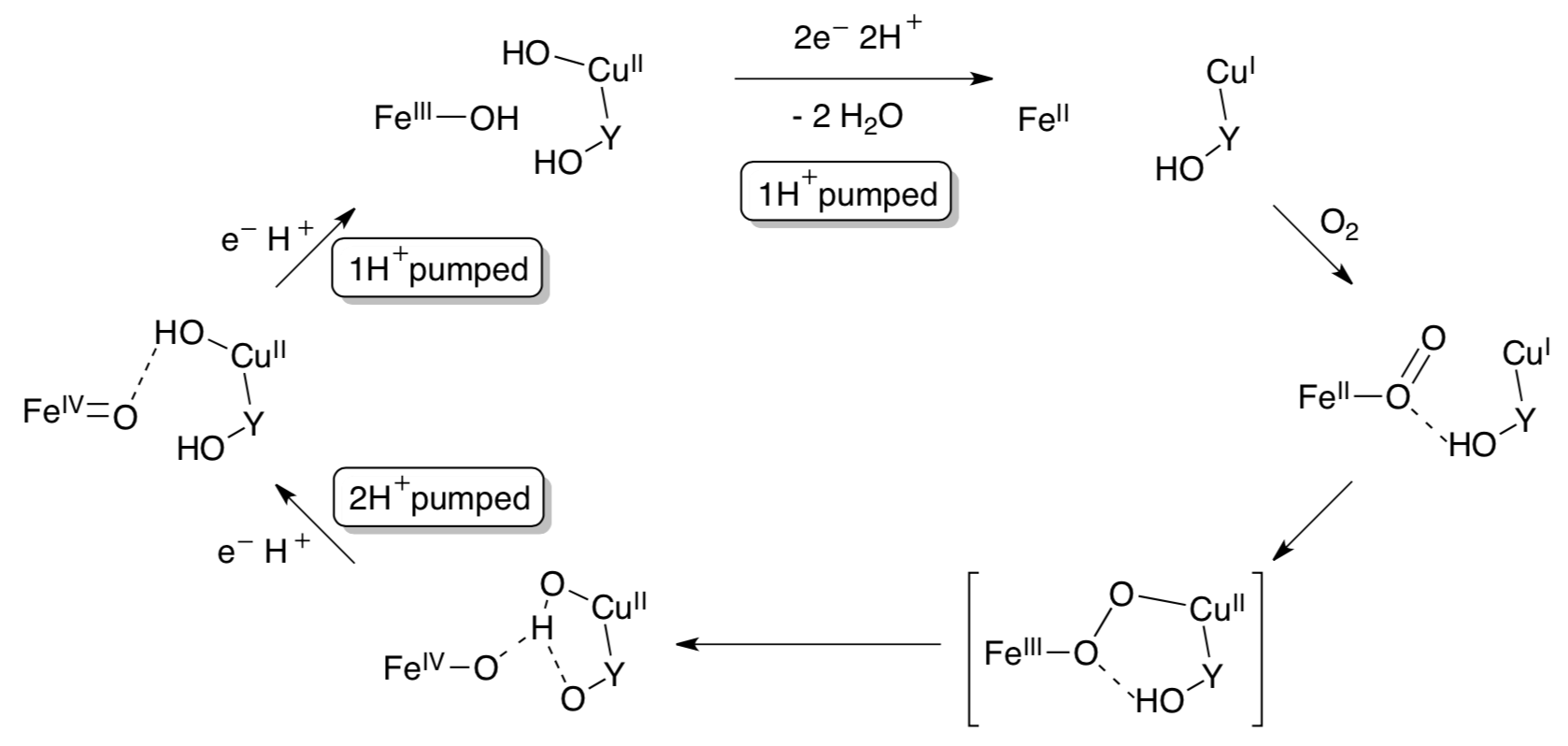
Wikström, M. (2000) Biochemistry, 239, 3515-3519 Brändén, Gennis, Brzezinski, Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2006, 1757, Pages 1052–1063
- Suggest an evolutionary reason for this unique Fe:Cu dinuclear cluster for the reduction of O2.
- In the electron transfer from heme a to heme a3 to dioxygen, what would be the consequence if dioxygen, dissociated from the heme a3 Fe before it were completely reduced?
In the next section, we will look at how electron transfer can power the proton pumping to the intermembrane space.
Redox Coupling of Electron Transport and H+ Transport
The key challenge has been to understand the redox coupling to H+ transport.
The formyl group on the heme a3 is coplanar with the heme in both oxidation states.
- How might this coplanarity affect the charge in the heme?
- Draw a resonance structure for this compound.
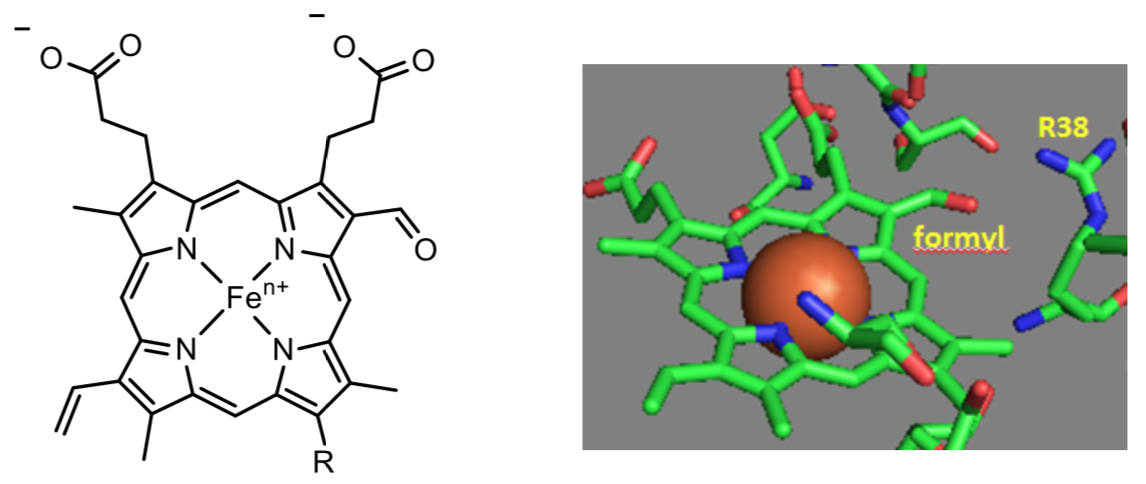
- From the figure above, what type of interaction would likely occur between Arg 38 (R38) and the formyl group?
- What might occur to the protonation state of adjacent protein side chains, specifically Arg 38 (R38) on reduction of the heme?
- How would this link electron and proton transfer?
H+ Transport Chain
The rapid exchange of H+ between H3O+ and H2O allows for a unique transport process known as the Grotthuss mechanism, which transfers protonic charge without diffusive movement of either an individual H+ or oxygen atom. This happens on a picosecond time scale.
Wraight, Biochimica et Biophysica Acta: Bioenergetics, 2006, 1757, 886–912.
- Use the diagram to explain how a proton might be pumped into the intramembrane space without diffusion.
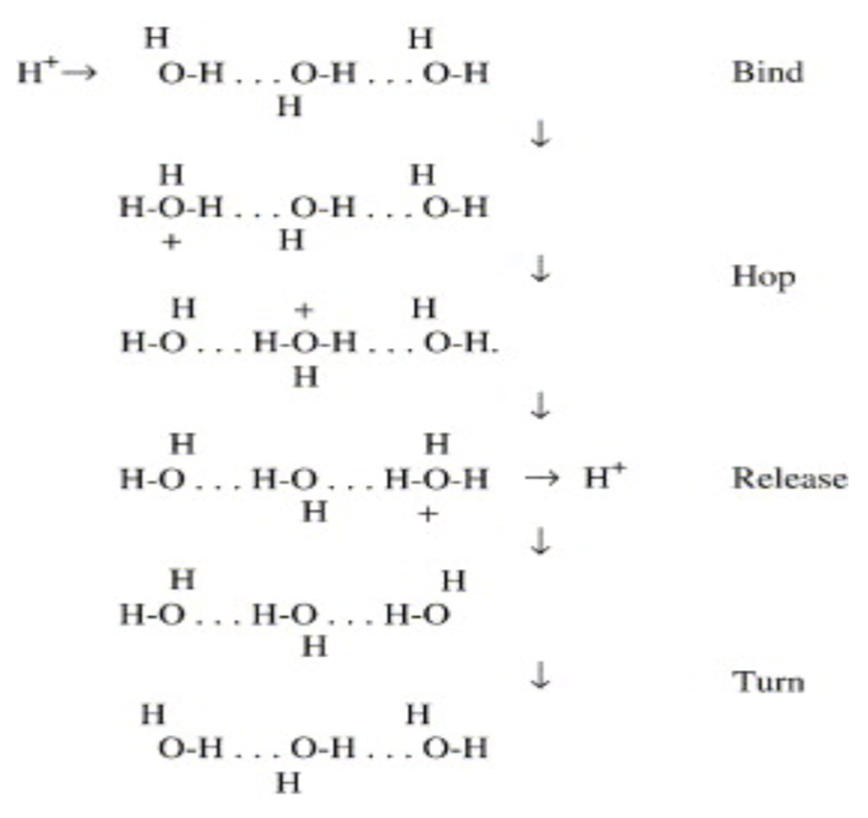
- What IMF is responsible for this movement?
- Is the H+ that starts through this process the same H+ released?
- Speculate on the rate of a proton being pumped via cytochrome c oxidase.
- Why would these complexes have evolved a proton transport mechanism using the Grotthaus mechanism instead of diffusion?
H+ Transport (D-path of Complex V)
H+ transfer does not occur by physical movement of an individual proton through a “proton pore”.
Crystal structures of oxidized and reduced CCOx show water channels and small “cavities” which calculations show can hold 1-3 water molecules. Hence groups around the heme, including R38 are accessible to water.
Ling Qin, Jian Liu, Denise A. Mills, Denis A. Proshlyakov, Carrie Hiser and Shelagh Ferguson-Miller,Biochemistry, 2009, 48 (23), pp 5121–5130
- What might be the function/role of the waters in the channel?
Some of the amino acid residues associated with the water channels are shown in the diagram below and include R38, S34, T 424, S461, S382, H413.
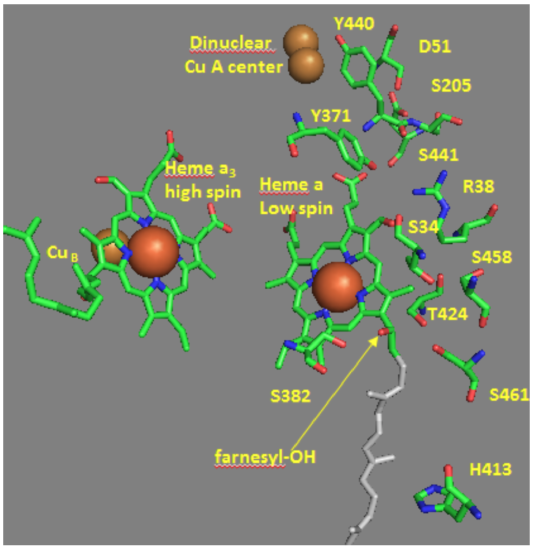
- Draw the functional groups from these amino acids.
- How might these amino acids be involved in proton transfer?
- Draw an arrow on the diagram showing the direction of proton flow.
D-path for proton transfer in Complex IV
Structural and functional studies show a key role for Asp 51 (D51). In the oxidized state, D51 interactions with two OH side chains and amide NH backbone groups but is not exposed to water. On reduction, D51 lies on the surface in an aqueous environment.
- Draw the proton transfer through the D-path/channel with this scheme.
- Circle the original H+ and put a box around the H+ that emerges.
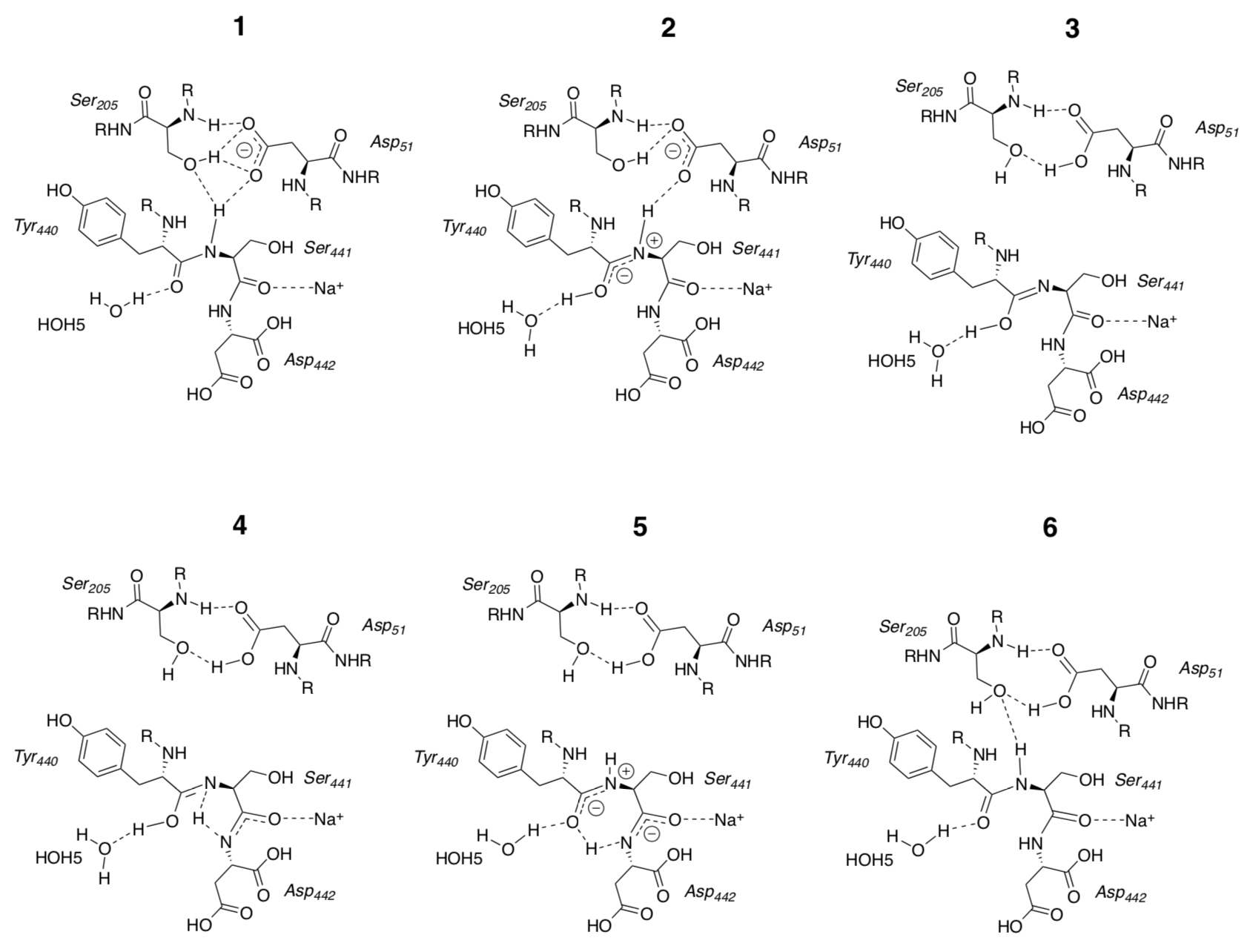
Kamiya, Boero, Tateno, Shiraishi, Oshiyama, J. Am. Chem. Soc., 2007, 129 (31), pp 9663–9673
- What is the end result for these structures? Are they regenerated?
Inhibition
Site specific mutagenesis has been used to change D51.
- What amino acid replacement might be optimal to affect activity but not protein folding?
The mutation uncouples electron and proton transport.
- Explain how this mutation “uncouples” the electron transport.
- What are the consequences of uncoupling electron and proton transport?
- To the number of ROS species produced?
- To ATP yield?
- One inhibitor of oxidative phosphorylation is CN- as it binds to the Fe-Cu complex more tightly than O2. What is the result of cyanide poisoning?
Not all inhibitors of oxidative phosphorylation are toxins.
- How much ATP would a hibernating animal need?
- In brown adipose tissue, regulated proton channels called uncoupling proteins can uncouple respiration from ATP synthesis. This rapid respiration produces ___________ to maintain body temperature instead of ATP for hibernating animals.
Summary of Complex IV
A final pathway for coupling electron and proton transport has been proposed.
- Use the information above to complete the following statements:
When heme a is oxidized, Arg-38 is mainly ____________ (protonated/deprotonated) and Asp 51 is ______________ (buried or exposed) and is ______________ (protonated/deprotonated).
Upon reduction of heme a, the net charge on heme a changes from _______ to ________.
This leads to _________ (increased exposure/decreased exposure) of Asp-51 to the ___________ (intermembrane space, matrix, membrane).
Coupled to this, protons on Asp-51 are ___________ (released to or taken up from) the intermembrane space.
On re-oxidation of heme a, Asp-51 moves back to the ___________ (interior/exterior) of the protein and the net positive charge on heme a ___________ (increases or decreases).
This leads to a _________ (increased or decreased) affinity of the heme formyl group for Arg 38.
This leads to proton transfer __________ (to/from) Arg 38 toward Asp 51.
Arg-38 then _____________ (gives up/take on) protons from water molecules in the water channel.


