18b: ComplexI
- Page ID
- 150555
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Complex I (NADH-quinone oxidoreductase)
Electron Transfer in Complex I
Complex I is located in the inner mitochondrial membrane in eukaryotes. The electrons from NADH (produced in the TCA cycle) begin to be shuttled through small steps to capture the energy.
This section will examine the mechanisms of electron transfer by the peripheral domain, proton transfer by the membrane domain and how their coupling can drive proton transport.
The net reaction of Complex I is the oxidation of NADH and the reduction of ubiquinone.
Net reaction: NADH + H+ + UQ -> NAD+ + UQH2
- Use the table of reduction potentials and the Faraday equation to calculate ΔG for this reaction.
The standard free-energy change ΔG° ́ is related the reduction potential ΔE ́0 by:
ΔG0' = -nFDE’0
F (Faraday’s constant) = 96,485 J/V mol
ΔE’0 = difference in reduction potentials between donor and acceptor
n = number of electrons transferred
Complex I
A cartoon model of the complex shows redox active cofactors in complex I. Complex I contains at least 46 different proteins and a number of prosthetic groups: a flavin redox agent (FMN), a ubiquinone redox agent (UQ) and some iron sulfide clusters (FeS).
The hydrophilic or peripheral domain catalyzes electron transfer while the membrane domain is involved in active transport of protons.
Nature 465 (2010) 441-447. doi:10.1038/nature09066Nature 465 (2010) 428-429.
Journal Biological Chemistry 284, (2009) 29773–29783. Journal Biological Chemistry 286 (2011) 18056–18065
Label the membrane-bound part of the complex.
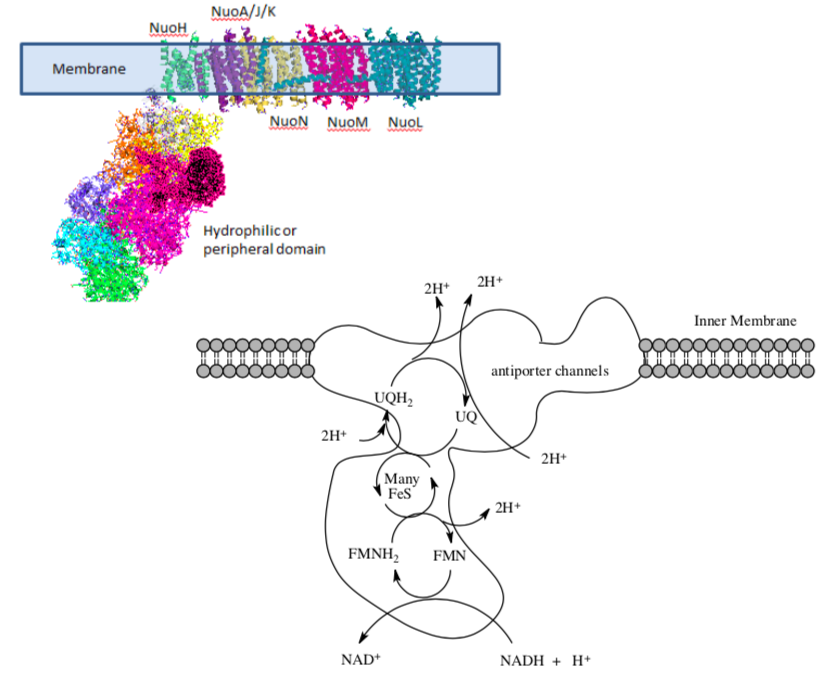
- Label the peripheral part of the complex (i.e. the other part).
- Electron transfer occurs more readily in polar environments. What amino acid side chains do you think are present in the peripheral region?
Follow the Electron Transfer through Complex I
Complex 1 and Close up views of FMN (a flavin redox agent) and Fe/S clusters are shown below where N2, N3, N4, N5, and N6 are tetranuclear FeS clusters. (N7 is found in some bacteria).
- Sketch in the possible site for NADH and ubiquinone (UQ) binding
- Indicate a possible path for flow of electrons from NADH to UQ.

- Given this path, what must be the relative relationships of the standard reduction potentials for FMN, the Fe/S clusters and ubiquinone for electrons to flow along the path.
- Are these inner sphere or outer sphere electron transfers?
- Why are there so many transfers?
Transfer 1: Electron transfer from NADH to FMN
The ultimate goal is to transfer electrons in baby steps in order to release energy incrementally, rather than all at once. The electron transfer chain uses Fe(II)/Fe(III) couples.
The first step in this process is the reaction of NADH with FMN.
The oxidation of NAD+ has been covered earlier:

FMN is a flavin redox cofactor like FAD).
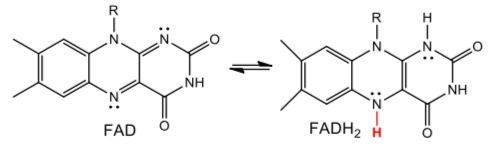
- How many electrons can FADH2 transfer at once? [ 1 OR 2 OR either]
- How many electrons can NADH transfer at once? [ 1 OR 2 OR either]
- Why doesn’t NADH transfer electrons directly to the iron? Assume that the FeS clusters can only accept one electron at a time.
- Why is a flavin redox center required as an intermediary?
Flavin cofactors are usually tightly bound to enzyme and are not released and rebound to the enzyme during the catalytic cycle.
- Why is it necessary for a flavin to be bound to the enzyme in Complex I?
Transfer 2: Electron Transfer from FMNH2 to Fe/S Clusters of Complex I
The electrons are transferred from FMNH2 to the FeS cluster.

The tetranuclear Fe/S cluster is based on the cubanestructure with Fe and S occupying alternating corners of a square in a tetrahedral geometry. Each Fe is also coordinated to thiolate anions. The actual structure is a distorted cube as shown below, along with that of the binuclear cluster, whose bond angle also deviate from those in a tetrahedron.

- What is the coordination number of the irons in the cubane ring?
- Assuming that all Fe are in the III oxidation state, what is the overall charge on the cluster?
- If one electron is added, what is the overall charge?
- Electrons are transferred from one FeS cluster to another in this pathway. Are these outer sphere or inner sphere transfers?
EPR studies suggest that not all of these electron transfers are exergonic, but rather the chain is like a roller coaster.
- Why does the system use a mixture of endergonic and exergonic electron transfers?
Transfer 3: Electron Transfer from FeS clusters to UQ

The Fe/S Clusters transfer single electrons to UQ one at a time.
The reduction of the ubiquinone (UQ) is shown below while the transfer of electrons from the FeS clusters to UQ is on the following page.
- Add the electrons to the UQ templates to show how UQ could be reduced in single electron transfers and protonation steps.

- Studies have shown that the ubiquinone radical is found only when bound to Complex I and not free in the membrane. This is true at many different overall ratios of UQ/UQH2. Why is this biologically important?
Mechansim of e- and H+ flow from FeS clusters to UQ
Electrons and protons flow from the bottom of each box to the top. Eventually there will be an increase in 2 e- and 2 H+ to be transferred to the UQ.
Berrisford and Sazanov, JBC, 284, 29773, 2009.
- Complete the diagram with correct charges and electron flow.

Complex 1 is done with its redox task; the UQH2 moves on to the next complex...
Summary: Proton Transfer by the Membrane Domain in Complex I
In the catalytic cycle of Complex I (earlier), protons are produced AND moved from inside mitochondria to a space between the membranes.

- How many protons are moved across the membrane for each catalytic cycle of Complex I?
- Is this active transport or passive diffusion?
- If this is active, what is fueling this transport?
- Is this with or against the concentration gradient? (i.e. antiporter or synporter?)
Application Problems
- Complex I is inhibited by more than 60 different families of compoounds. They include the classic Complex I inhibitor rotenone and many other synthetic insecticides/acaricides. The classes include: Class I/A (the prototype of which is Piericidin A), Class II/B (the prototype of which is Rotenone) and Class C (the prototype of which is Capsaicin). They appear to bind at the same site.
- From the structure of the 3 prototypes, what are the characteristics of the pharmacophore, the “ideal binding ligand”?
- Where do they likely bind?
- How “promiscuous” is the binding site?

- ManydevastatingneurologicaldiseaseslikeParkinson’sDiseaseareassociated with defects in Complex I. In addition to major problems with oxidative ATP production, reactive oxygen species (ROS) increase. The major sites for generation of ROS are Complex 1 and Complex III. Given the locations of the electron carriers at the periphery and internal within the protein complex, which electron carriers might most readily leak electrons to dioxygen? What ROS is likely to form in the process?
- InhibitorsmightblockaccessofUQorconformationalchangesnecessaryfor final reduction of the ubiqinone free radical. Class A inhibitors dramatically increase ROS production. The actual site of ROS production in Complex is a bit controversial.
- One possible electron donor to dioxygen is FMN. Why is this a likely candidate?
- Mutants that lack N2 iron-sulfur cluster showed ROS production. Is this consistent with FMN site involvement in ROS production?
- Basedonthestandardmidpointreductionpotentials,whichmightbethebest candidate to promote dioxygen conversion to superoxide (not to water), FMN or N2?
- Aparticularinhibitor,DPI,inhibitedROSproductioninthepresenceofexcess NADH but enhanced it in the presence of high UQH2 (which could allow reverse electron transport. Does this suggest FMN or N2 involvement in ROS production?

- DPI inhibition requires NADH. The higher the NADH/NAD+ ratios, the high the inhibition. At high NADH levels the FeS clusters stay oxidized.
- Must the protein be reduced or oxidized for interaction with DPI?
- IfcomplexIisisolatedfromthemitochondriaandinsertedintoartificial membranes, superoxide is produced in a slow step (along with a bit of H2O2). In a fast process, the following disproportionation (self-redox) can occur: O2- + O2- + 4 H+ -> H2O2 + O2, allowing superoxide production to be monitored by measuring peroxide levels. As above, the rate of superoxide production depends on the NADH/NAD+ ratio.
- Hence what must be bound at the active site for superoxide production to occur?
- In intact mitochondria, if a Q site inhibitor like rotenone is added, superoxide production increases. What would Q site inhibitors do to the levels of NADH?
- In submitochondrial preparations,normal Complex I activity occurs (which leads to formation of a sustained proton gradient). Also reverse electron transport, powered by an artificial proton gradient can occur, which leads to the reduction of NAD+ can occur (see diagram below).

A summary of finding on superoxide production by Complex I is given below:
- Superoxide production is inhibited flavin site inhibitors but not Q site inhibitors.
- Reverse electron transport leads to NAD+ and O2 reduction
- Reverse electron transport superoxide production is inhibited by both flavin and Q site inhibitors
- Based on these findings, which site (the flavin or Q site), is involved in superoxide production.


