17: Mass Spectrometry
- Page ID
- 150553
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCFragmentation in Mass Spectrometry
Mass spectrometry bombards a molecule to cause the loss of an electron. The radical cations (molecular ions) quickly decompose via radical reactions.
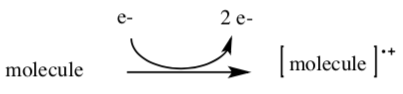
The mass spectrometer can provide a molecular mass for any positivelycharged molecular ion or fragment.
Inductive Cleavage
- Show loss of an electron from the following molecule. Also add arrow(s) in the second step.

- Circle any ions that would be observed.
- What is the mass of the product(s) observed?
- Why does a bond break easily after this ionization?
Inductive cleavage is also possible in the following pathways.
- Show the results of the first ionization and show the inductive cleavage that results, with arrows.

- In each case, give reasons why the bond broke easily.
Alpha Cleavage
- Show loss of an electron from the following molecule.
- Also add arrow(s) in the second step.

- Circle any ions that would be observed.
- What is the mass of the product(s) observed?
- Why does a bond break easily after this ionization?
Alpha cleavage is also possible in the following pathways.
- Show the results of the first ionization and show the alpha cleavage that results, with arrows.
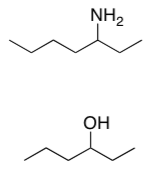
- In each case, give reasons why the bond broke easily.
McLafferty Rearrangement
- Show loss of an electron from the following molecule.
- Also add arrow(s) in the second step.
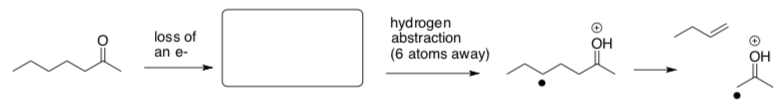
- Why does the molecule rearrange after this ionization?
- What is the mass of the cationic fragment? Is it even or odd?
- What is the mass of the cationic fragments from alpha cleavages? Are they even or odd?
McLafferty rearrangement is also possible in the following pathway.
- Show the results of the first ionization and show the inductive cleavage that results, with arrows.
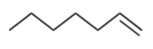
- What structural requirements are necessary for a McLafferty rearrangement?
Sigma Cleavage
- Show loss of an electron from the indicated sigma bond in the following molecule. Why does that bond then break easily?

- What is the molecular weight of the ion that results?
- Why is loss of an electron from a sigma bond less likely in the first place?
- Although impossible in the molecule shown above, what electrons are most likely to be lost in the initial ionization in mass spectrometry?
Additional Problems
- Analyzethefollowingspectrumof4-heptanone.
- Draw the structure of 4-heptanone.
- Show a mechanism for an alpha fragmentation and the McLafferty Rearrangement/Fragmentation for each compound.
- Use your predicted fragments to match the peaks within the MS.
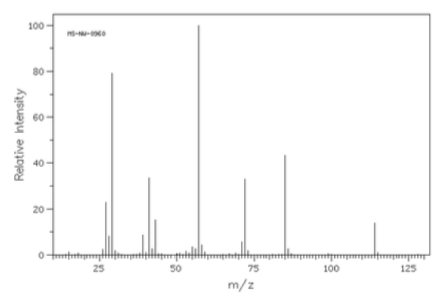
- Compounds X and Y are isomers with the formula C3H6O. Compound X has a peak at 1730 cm-1 in the IR and Y has a peak at 1715 cm-1. The spectra are shown below. Determine the structures of Compounds X and Y.
Compound X
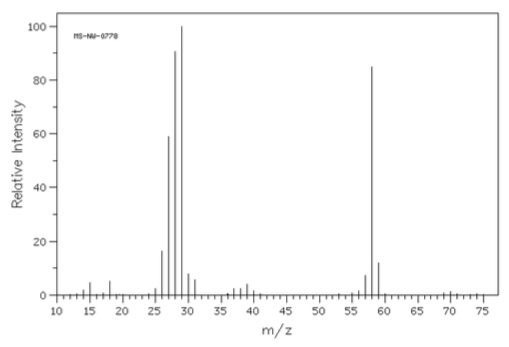
Compound Y
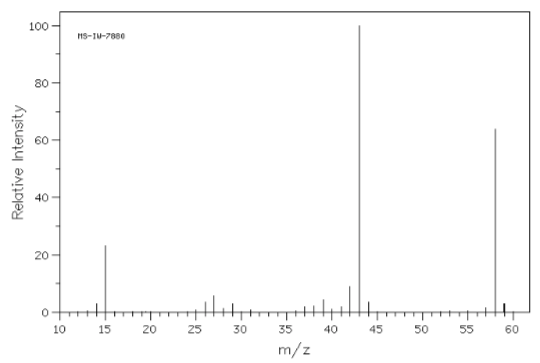
- The mass spectra of three isomeric amines (sec-butylamine, isobutylamine and tert-butylamine) are shown below.
- Draw these three amines.
- Show all possible a-fragmentations.
- Match each spectrum with the correct structure.
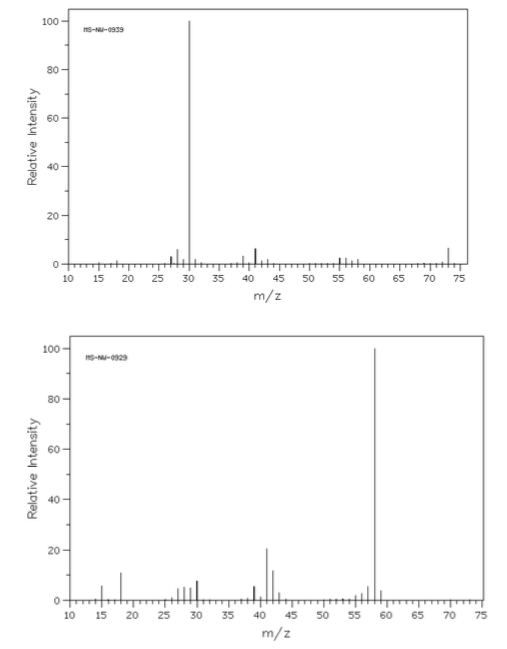
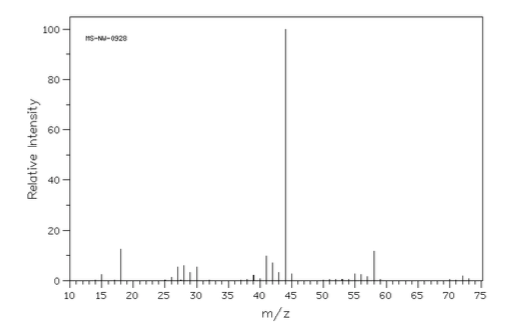
- Match the following mass spectra to the expected products. For each show how the assigned peaks were generated.
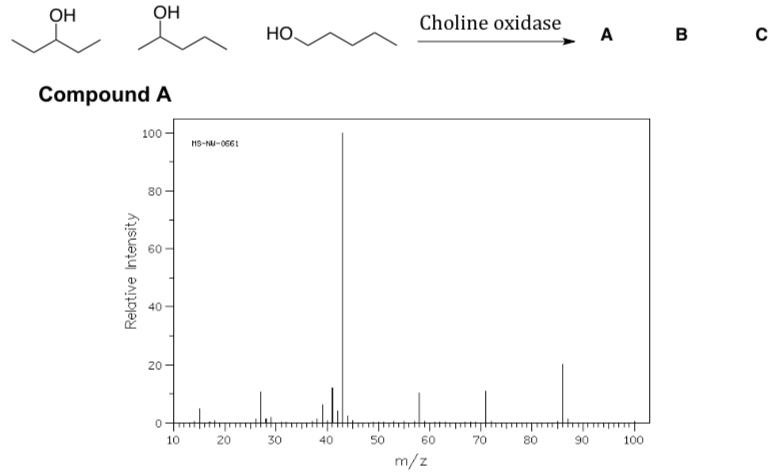
- Show fragmentation that results in the peak at m/z = 43:
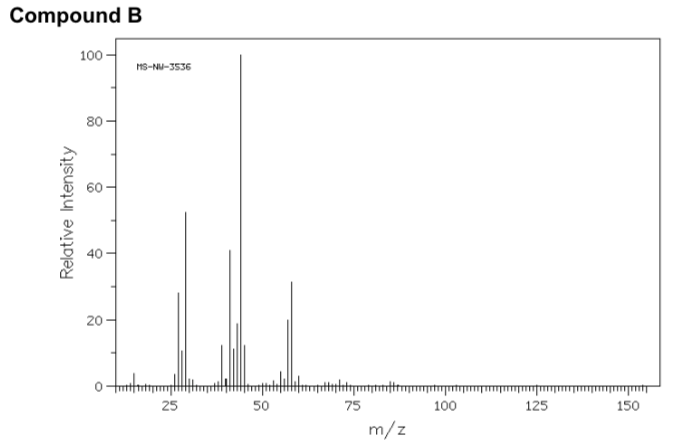
- Show fragmentation that results in the peak at m/z = 44:
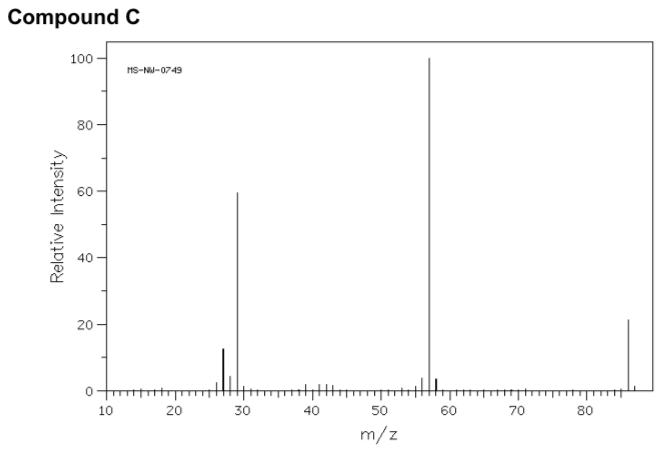
- Show fragmentation that results in the peak at m/z = 57:
Cumulative Radical Application Problem
Nitric oxide synthases (NOSs) are a family of enzymes catalyzing the production of nitric oxide (NO) from L-arginine. They are closely related to the cytochrome P450 family of enzymes. NO is an important cellular signaling molecule. It helps modulate vascular tone, insulin secretion, airway tone, and is involved in angiogenesis and neural development.
JBC 2010, 285, 7233-7245.
The following is the overall reaction:
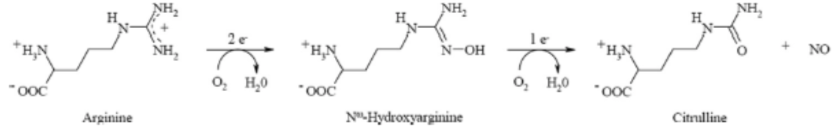
- Draw a Lewis structure for NO
- Provide arrows for the following activation of O2 by cytochrome P450 (not all steps require arrows).
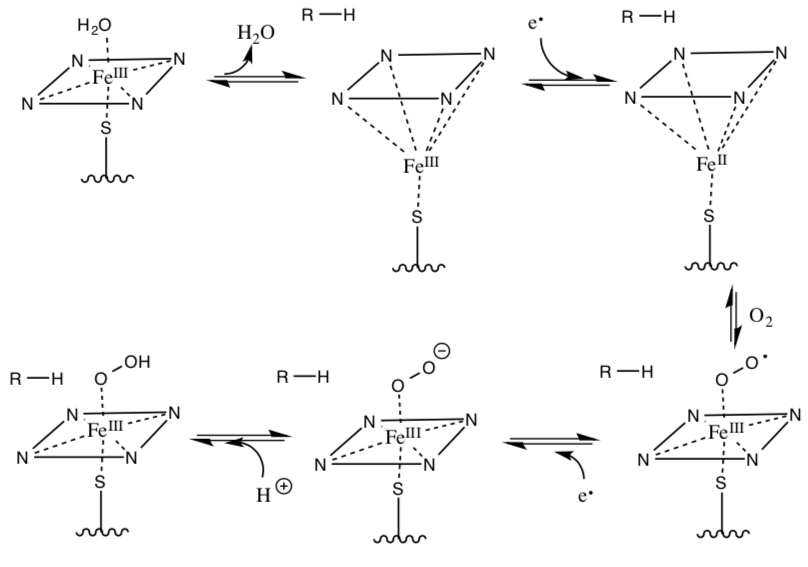
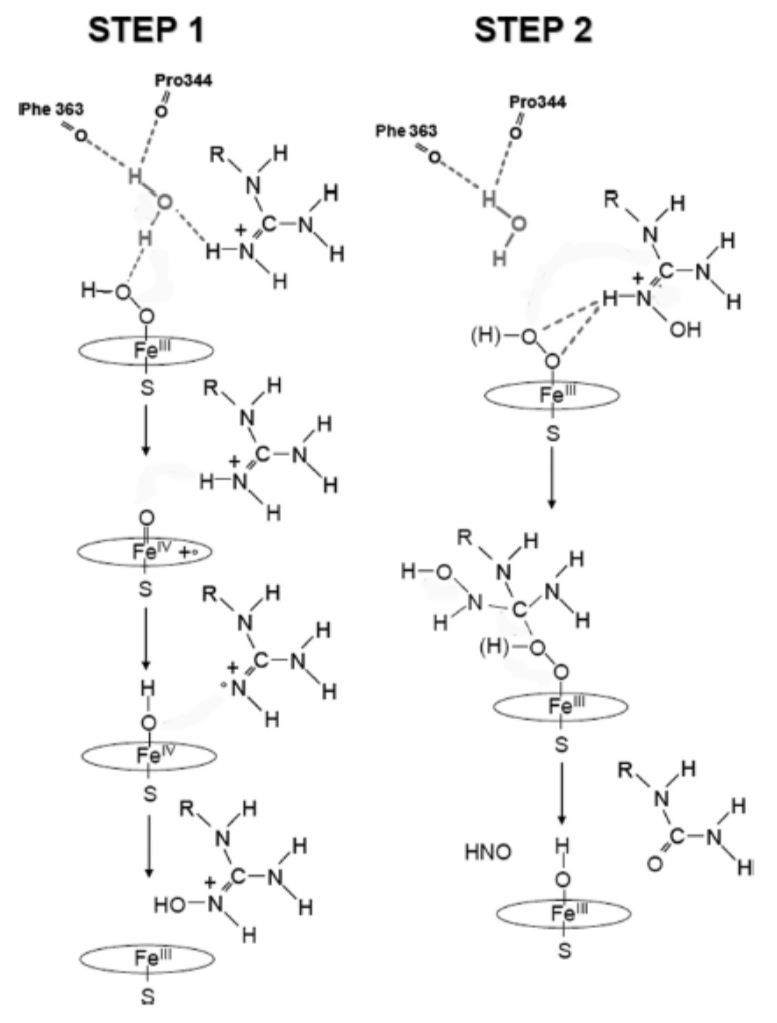
- Below are the two steps for the NOS mechanism using the activated O2/iron species. Provide arrows for the following mechanism:
- Draw a splitting diagram of an octahedral Fe3+ complex and predict the spin-only magnetic moment
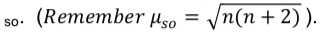
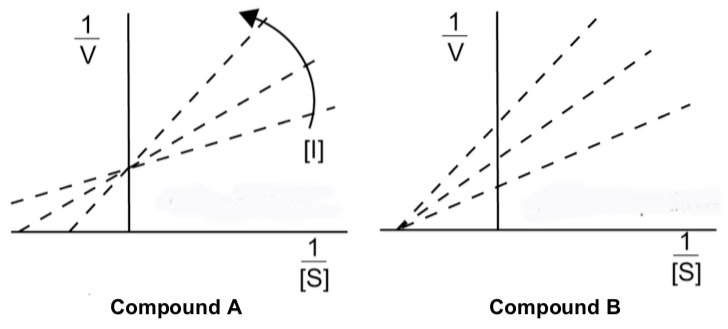
- Compounds A and B were synthesized as inhibitors to NOS. They both displayed different types of inhibition. Look at the following data and decide what type of inhibition each is exhibiting.
- The following mass spectrum was obtained for compound B. Provide a mechanism for the formation of the base peak at m/z = 57
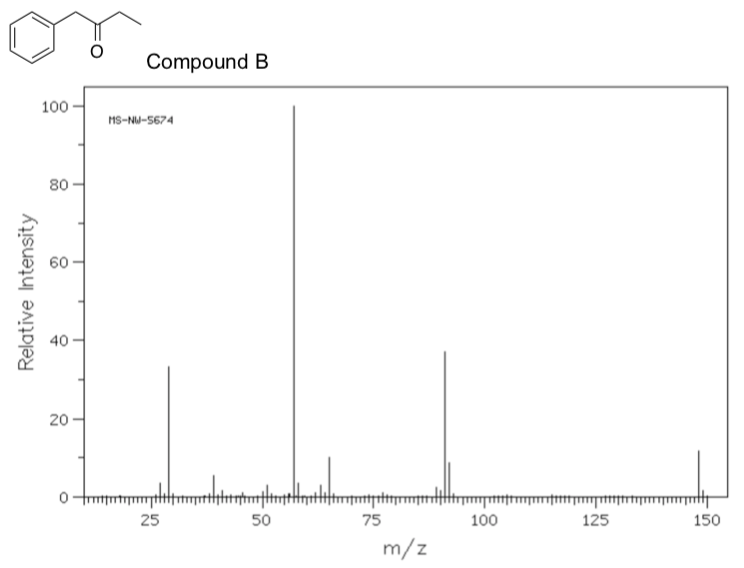
- What is the IUPAC name for compound B?


