16: Biological Radical Reactions
- Page ID
- 150550
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Biological Radical Reactions
Ribonucleotide Reductases
Ribonucleotide reductases (RNRs) are enzymes that convert ribonucleotides to deoxyribonucleotides, which are building blocks of DNA. Different organisms have different RNRs that react via different mechanisms, but the simplified scheme shown below is representative of the intermediates that have been detected in the pathway.
- Provide reaction arrows in the scheme below.
Note: PP = diphosphate, B = base, Xyz = amino acid abbreviations
Hot Potato!
Some of the amino acids surrounding the ribonucleotide, such as the nearby glutamic acid (Glu) and aspartamine (Asp) may not actively participate in the chain reaction.
- Suggest a role for these amino acid residues.
- Why is a hydrogen lost from the neighboring carbon first, before the hydroxyl group is lost?
Oxidizing Agents: Dioxygen
O2, dioxygen, is one of the most potent oxidizing agents used in nature.
- Draw the Lewis structure of dioxygen, O2.
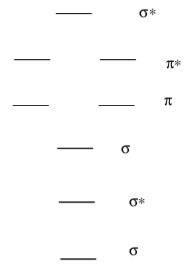
- Fill in the MO diagram for O2.
- Suggest how O2 might be able to participate in radical reactions.
If dioxygen acts as an oxidizing agent, it can get fully reduced to water.
- As dioxygen is reduced, it (gives electrons/accepts electrons). Circle one.
- Balance this equation:
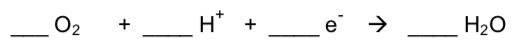
This reaction really happens one electron at a time.
- Draw Lewis structures for the following series of oxygen species (superoxide, O2-; peroxide, O22-; oxide, O2-).

Properties of Dioxygen
Chemically, cellular respiration is considered an exothermic redox reaction.
Technically, cellular respiration is a combustion reaction but it clearly does not resemble one when it occurs in a living cell.
C6H12O6 + _____ O2 -> _____H2O + _____CO2
- Balance the above reaction.
- Show the two half-reactions.
Nature must deal with these two properties of dioxygen so as to utilize oxygen in the oxidation of carbohydrates and lipids to CO2 – without a combustion!
- How does an organism complete the oxidation of glucose without a fire?
- How does an organism activate the oxygen (despite its lack of reactivity)?
- How does an organism transport O2?
As oxygen reacts as an oxidizing agent, it gets reduced to produce nasty, dangerous products (superoxide, peroxide).
- Could you imagine a situation when we would want to produce these species?
PUFA radical oxidation
Ground state triplet oxygen reacts readily with certain fats in the body.
Oxidation of PUFAs is mediated by free radicals such as the hydroxyl radical (HO•). Shown here is the proposed first step in the oxidation of linolenic acid, an w-3 fatty acid:
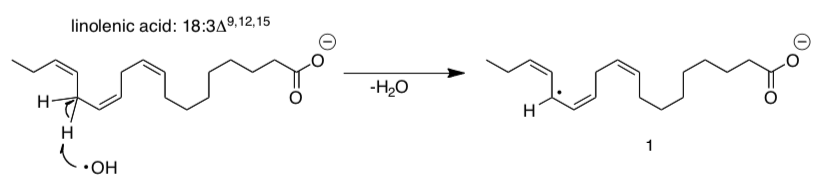
Although the reaction illustrated shows one of the hydrogens on C-14 being abstracted there are other hydrogen atoms that are susceptible to this kind of reaction.
- Draw resonance structures for radical intermediate 1.
- What can you conclude about the stability of radical intermediate 1?
- Identify the other hydrogen atoms that are also likely to be abstracted. [HINT: think about the stability of radical intermediate that is formed]
Radical intermediate 1 reacts with molecular oxygen:
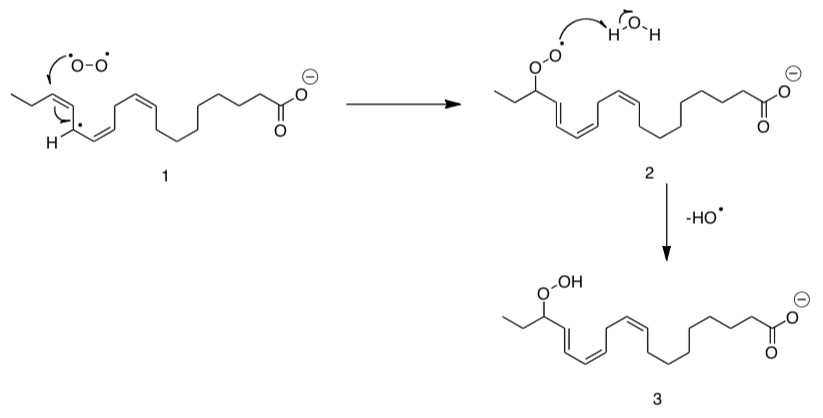
- How does this mechanism allow for propagation of this radical degradation process?
Oxidizing Agents: NAD+ and FAD
NAD+ and FAD are enzyme cofactors that are used in some biological redox reactions.
- Show arrows for the following redox reactions using FAD.
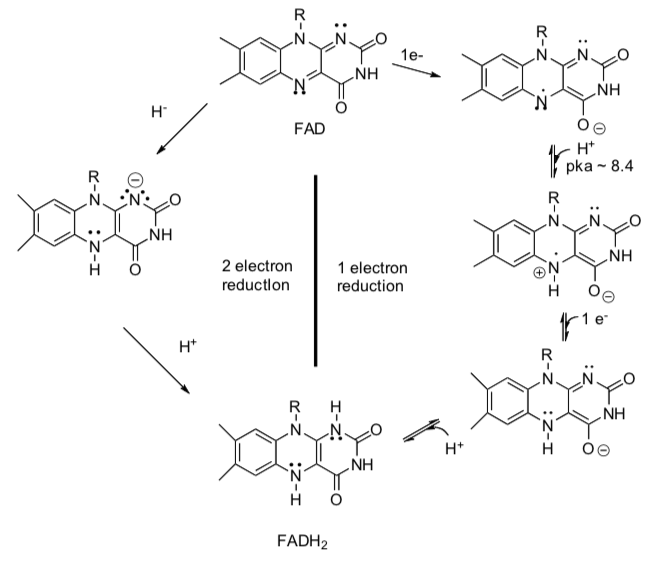
- Show arrows for the following redox reactions using NAD+.
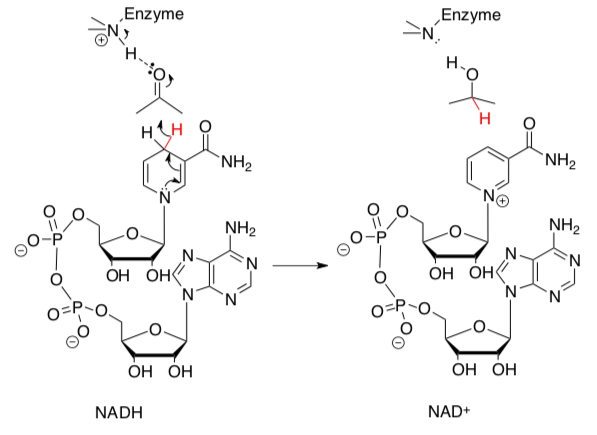
The reactive sub-structures of NAD+ and FAD are shown below.
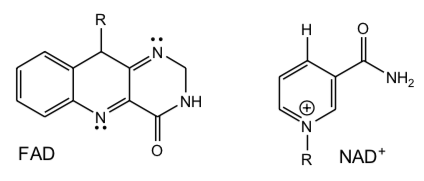
- Explain why FAD can accept either 1 or 2 electrons but NAD+ only two when they act as oxidizing agents.
- Thus, which is more likely to react with O2 in solution (no enzyme present), FAD/FADH2 or NAD+/ NADH redox pairs? Why.
Consider the reactions below:
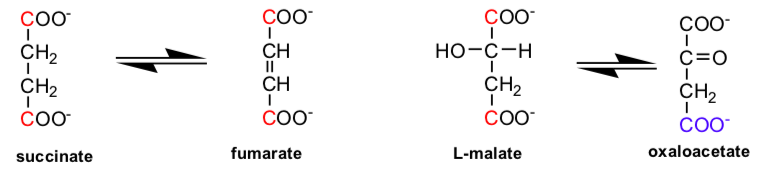
Standard Reduction Potentials
| oxidant | reductant | n (electrons) | Eo' (volts) |
| NAD+ + 2H+ | NADH + H+ | 2 | -0.32 |
| Oxalacetate + 2H+ | malate | 2 | -0.17 |
| Fumarate + 2H+ | succinate | 2 | 0.03 |
| FAD + 2H+ (bound) | FADH2 (bound) | 2 | 0.003-0.09 |
| 1/2 O2 + 2H+ | H2O | 2 | 0.816 |
- What type of reactions are these?
- Which is thermodynamically more difficult to oxidize, malate or succinate?
- Which is a more powerful oxidizing agent, NAD+ or FAD?
- Which oxidizing is probably the choice for thermodynamically difficult oxidation reactions, NAD+ or FAD?
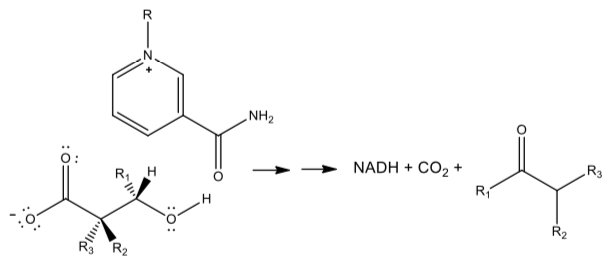
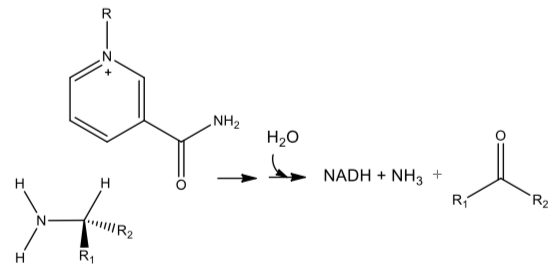
NAD+ in Redox Enzymes
NAD+ can be used in several other oxidative reactions.
- Draw mechanisms which show how it might be used for the following reactions. Assume general acids (BH) and bases (B:) are available in the enzyme active site:
Oxidative Decarboxylation
Oxidative Deamination
Biological Redox Enzymes
There are many types of enzymes that catalyze oxidation reactions using a variety of oxidizing agents.
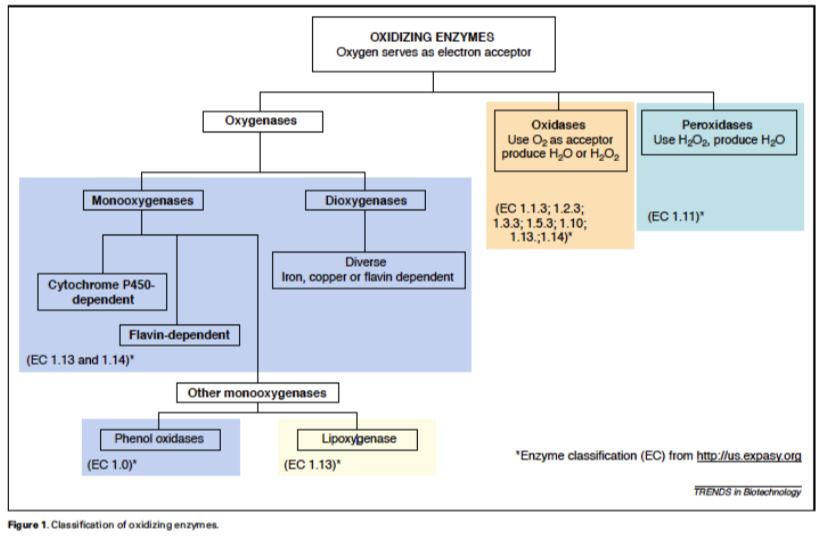
Stephanie G. Burton, Oxidizing enzymes as biocatalysts, Trends in Biotechnology, Volume 21, Issue 12, December 2003, Pages 543-549, ISSN 0167-7799, http://dx.doi.org/10.1016/j.tibtech.2003.10.006.
(http://www.sciencedirect.com/science...6777990300283X)
PASSPORT:
- Use the link above to answer questions about the following redox enzymes.
a) Monooxygenases; b) Dioxygenases; c) Oxidases & d) Peroxidases
- How do these enzymes vary?
- What is the actual oxidizing agent?
- Provide a sample reaction for each type of enzyme.
Metal Oxo Complexes
Active metal oxo species (i.e. containing an M=O fragment) play a role in a number of oxidation reactions in biology and industry.
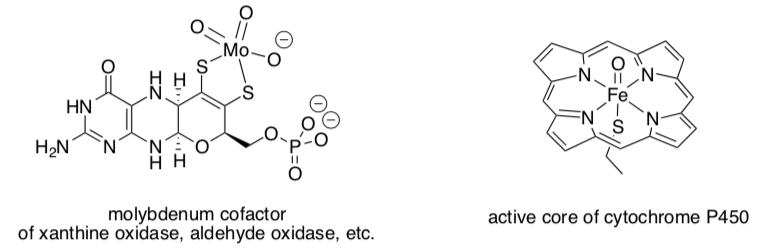
There is strong evidence for very reactive d4 or d3 oxos in biology. However, the vast majority of examples of metal oxos are less reactive and involve d0 or d2 metal ions.
Bonding in Octahedral Metal Oxos
Let’s look at the bonding in oxos to see why they behave in different ways according to the electron count on the metal.
- Why do atoms fill in the order 3p, 4s then 3d in the periodic table (why is d extra high in energy)?
- In ions, the order reverts to “normal” because the 3d orbitals contract and drop in energy. Why do they contract more than the 4s or 4p orbitals?
- Construct an MO diagram that describes donation from sigma bonding ligands. Put a box around the part that we call “the d orbital splitting diagram”.
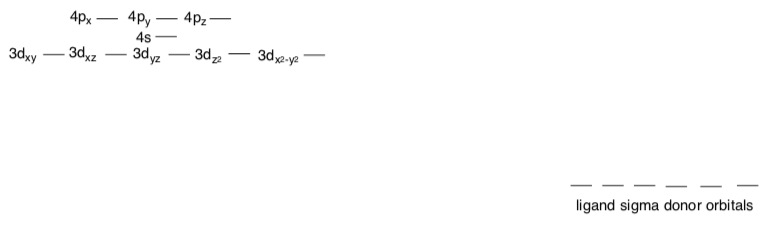
Pi Donation in Octahedral Metal Oxos
- Suppose the oxo ligand is along the z axis. Circle the p orbital(s) that could be used to pi donate to the M.
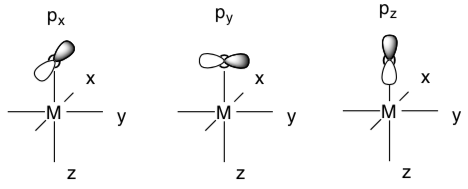
- Which of the t2g metal orbitals could it pi donate into?
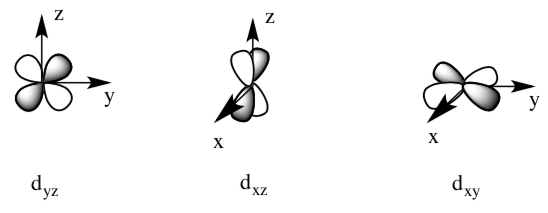
- Sketch pictures of the two pi bonds.
Splitting Diagram in Octahedral Metal Oxos
Here is the new splitting diagram to reflect pi donation from the oxygen.
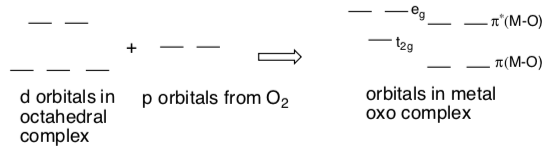
d0 and d2 oxo complexes relatively common.
- Fill in the pi-adjusted d orbital splitting diagram for d0 and d2 oxo complexes. Remember these diagrams should reflect the number of d electrons plus the 4 e- donated from the oxygen for the pi donation.
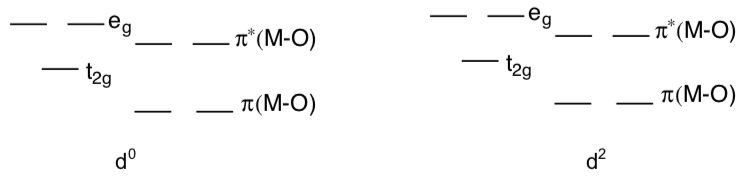
- Why are d0 and d2 oxo complexes relatively common? Explain with the help of the pi-adjusted d orbital splitting diagram, above.
- Why are d4 oxo complexes relatively reactive? Explain their reactivity with the help of a d orbital splitting diagram.
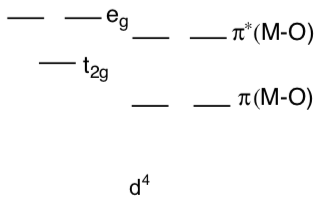
Fe(IV)=O is a common intermediate in biological oxidations.
Fe(IV)=O is d ________.
Quantum mechanical calculations indicate that the  orbital of a Fe(IV) oxo can have a significant amount of oxygen p character (over 40%; see Que, Inorg. Chem. 2009,48, 11038-11047).
orbital of a Fe(IV) oxo can have a significant amount of oxygen p character (over 40%; see Que, Inorg. Chem. 2009,48, 11038-11047).
This data could be interpreted in the following two resonance structures:
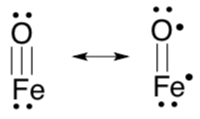
- The significance of this finding is that the unpaired electron is located... on the Fe atom / on the O atom / equal probability on Fe or O atom (circle one option).
- What type of reactivity would the oxo initiate? Complete the following example with curved arrows.
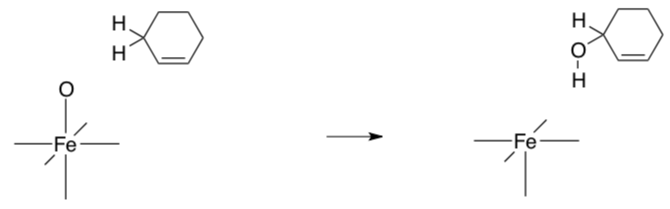
- How stable is the Fe(IV) complex?
- Would you expect any transition metal-oxo complex to form with transition metals with more than 4 d electrons?
Metal Oxygenases
Taurine Dioxygenase
Oxygenases facilitate the 4-electron reduction of O2.
- Add electrons and protons to balance the following equations:
O2 -> 2 H2O
O2 + R-H -> H2O + R-O-H
Nonheme iron oxygenases tend to be very specific. They generally utilize a cofactor. For example, taurine: a-ketoglutarate dioxygenase relies on a-ketoglutarate. (Riggs-Gelasco, J. Am. Chem. Soc. 2004, 126, 8108-9.)
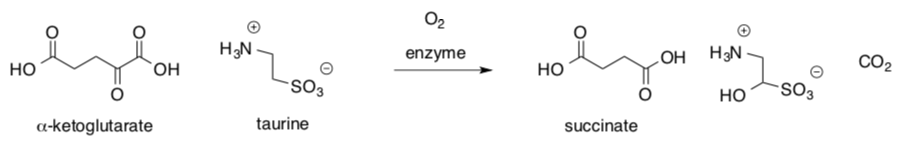
- Explain how a-ketoglutarate acts as a reducing agent in the reaction.
- Use arrows to keep track of electrons in this proposed mechanism.
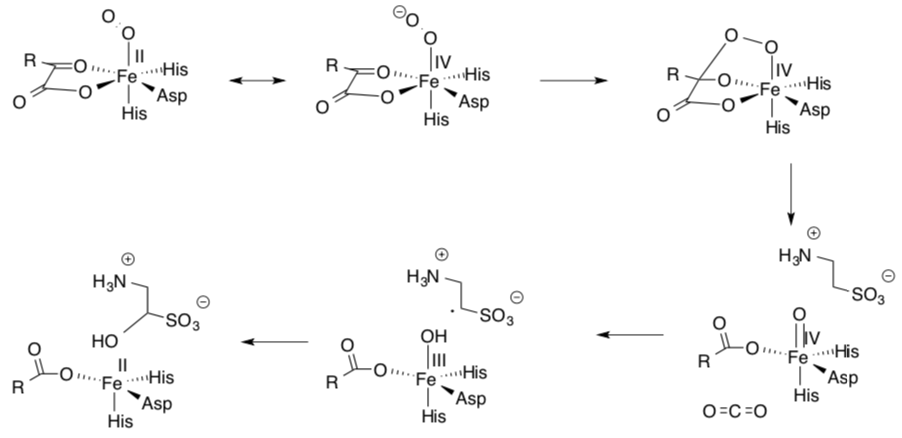
Pamela Riggs-Gelasco and collaborators support this mechanism with X-ray absorption spectra that suggest Fe(IV) intermediates are present.
Heme Dependent Monooxygenases
Heme enzymes contain a protoporphyrin ring with a bound Fe3+ ion. Cytochrome P450 are an example of heme enzymes and they are important enzymes in the hydroxylationxenobiotics, that is, drugs and other chemicals foreign to living organisms.
- Why would your body want to introduce an alcohol functional group on an alkane of a drug or other toxin?
The function of the heme is to activate molecular oxygen to species that can insert into various molecules.
- What is the shape of the following iron complex?
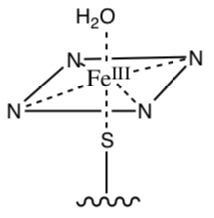
- Draw the splitting diagram for this metal and fill in the electrons (Fe3+ is low spin).
When the substrate (R-H) enters the active site the top ligand (H2O) is released and the iron adopts a five coordinate high spin state in which the iron is out of the plane of the porphyrin ring.
- What is the new shape of the complex?
- Draw a splitting diagram for this metal and fill in the electrons.
Cytochrome p450 (cont.)
Conversion from low spin to high spin state results in a large increase in redox potential of the heme (from -330 mV to -173 mV).
- This change in reduction potential means that the iron to more readily (donate or accept) an electron. Propose a reason based on the splitting diagram.
Molecular oxygen binds to the ferrous (Fe2+) heme to give the low spin peroxy radical and then another electron is transferred to give the heme peroxy anion.
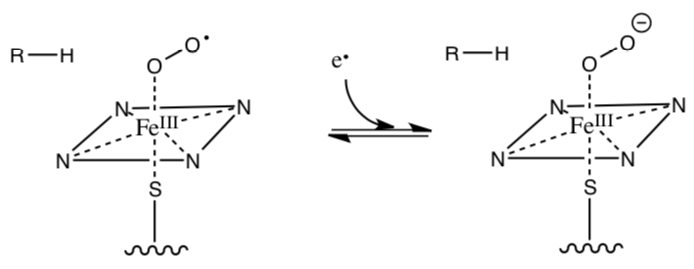
- Is this electron more likely to come from NAD or FADH2?
- How likely is the peroxy anion to be protonated?
- The next step is the generation a very high energy oxo ferryl heme complex (shown below). Provide a mechanism for this.
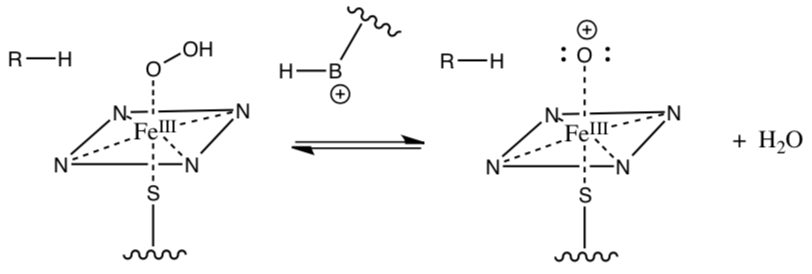
Cytochrome p450 (cont.)
The high energy oxo ferryl heme complex can be stabilized by resonance by transferring an electron from the iron to the oxygen (there are also several other resonance structures postulated, but we won’t go into those).
- Draw the resonance structure described above.
This intermediate reacts with the substrate to hydroxylate it. Provide the missing arrows to the mechanism.
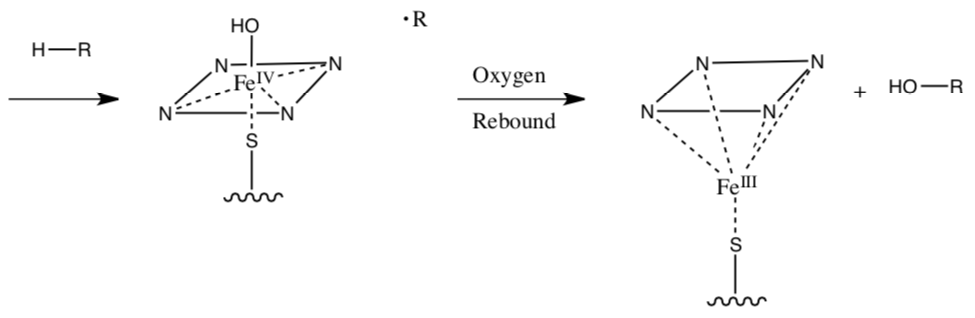
Evidence for this mechanism comes from substrate specificity for the enzyme. The reaction occurs preferentially at the more substituted (tertiary > secondary > primary) carbon.
- Why is the substrate specificity “good evidence” for this mechanism?
Cytochrome p450 (cont.)
- Provide arrows for the following activation mechanism (not every step requires an arrow):

Cytochrome p450: A Heme Monooxygenase
The cytochrome p450 enzyme can catalyze the difficult oxidation at a specific carbon in aliphatic hydrocarbons and in a variety of other molecules.
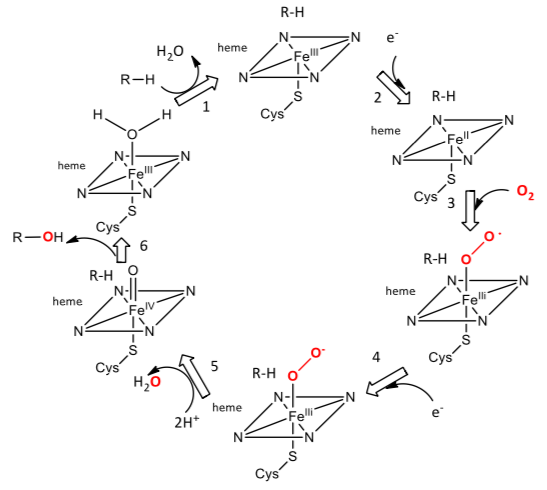
Step 1. The cleavage of the peroxide on water formation leaves one oxygen attached to the heme. It is this O that inserts into organic substrates. The best evidence indicates that it is an Fe(IV)-oxo-porphyrin cation radical.
- Draw the reaction of this cation radical with R-H substrate to initiate its hydroxylation.
Cytochrome p450 (cont.)
The reaction of P450 with camphor was studied by UV/Vis spectroscopy.
- What is the meaning of the constant 3.1 x 105 M-1s-1? For the oxidation of lauric acid the constant was kapp = 1.1 × 107 M-1s-1.
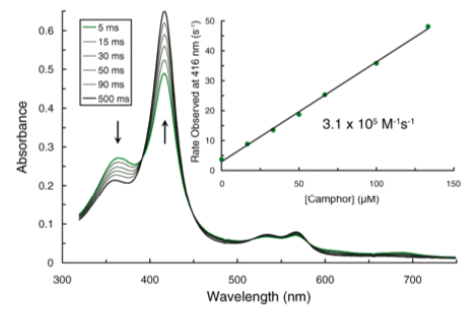
- What role might cytochrome P450 have in catabolism of nonpolar hydrocarbon molecules? CYP450 is involved in the initial catabolism of up to 75% of pharmaceutics. In anabolic reactions forming secondary metabolites? Given examples of each.
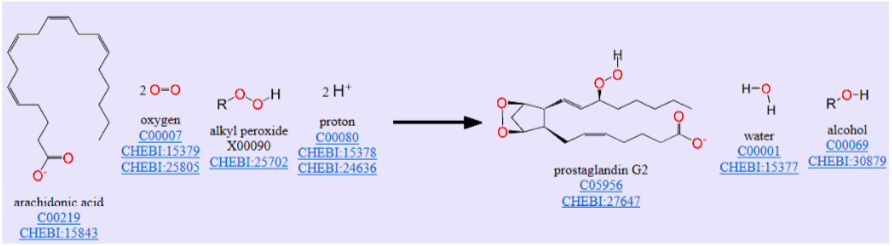
Cyclooxygenase: A Heme Dioxygenase
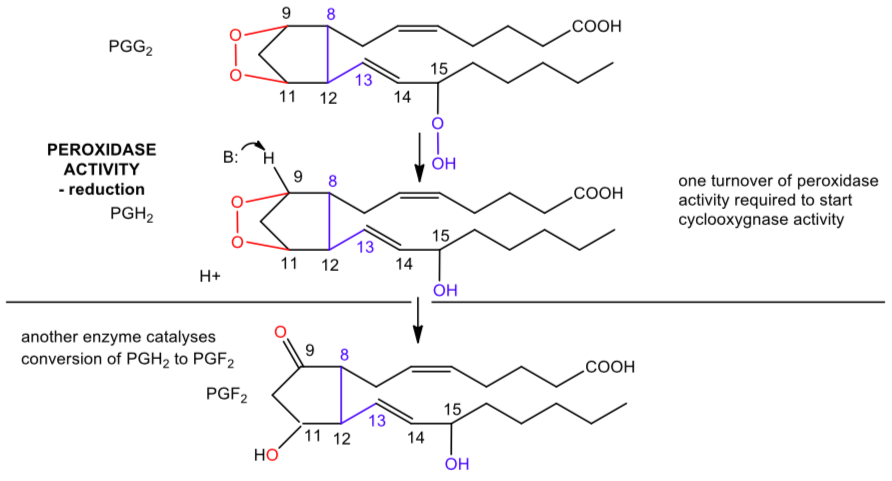
An example of a dioxygenase is cyclooxygenase, which catalyzes the reaction of arachidonic acid to prostaglandin G2 as shown in the overall reaction below.
A structure of arachidonic acid in the active site of the enzyme is shown below in the stereoimages. Stereo image of the experimental difference electron density map before the inclusion of AA (green trace) or PGH2 (gold trace) b, Diagram of PGH2 (gold) and AA (cyan) bound to the cyclooxygenase active site. Mobile side chains are colored gold and cyan to match their PGH2 and AA bound positions, respectively.
- Is arachidonic acid an ω-3 or an ω-6 fatty acid?
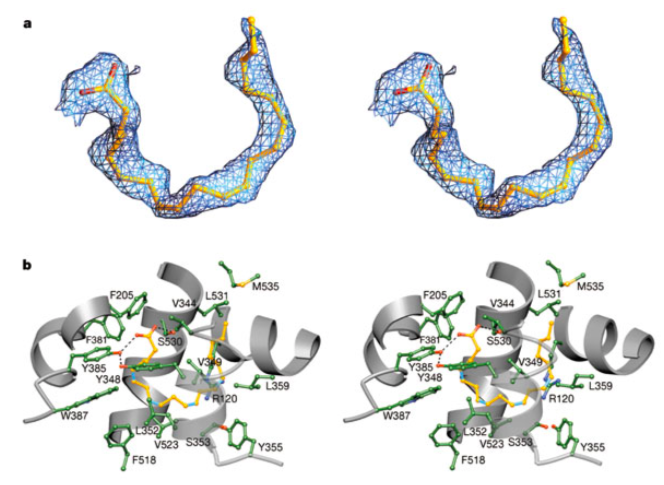
- Why is it bound in such a bent fashion in the structure shown?
Cyclooxygenase (cont.)
This enzyme actually catalyzes two different activities, catalyzing both a cyclooxygenase and peroxidase reactions. A possible free radical mechanism for the enzyme-catalyzed reaction cyclooxygenase part is shown below.
- From the name cyclooxygenase, what might happen to an oxygen reactant?
- Add curved arrows to the reaction to complete the reaction mechanism.
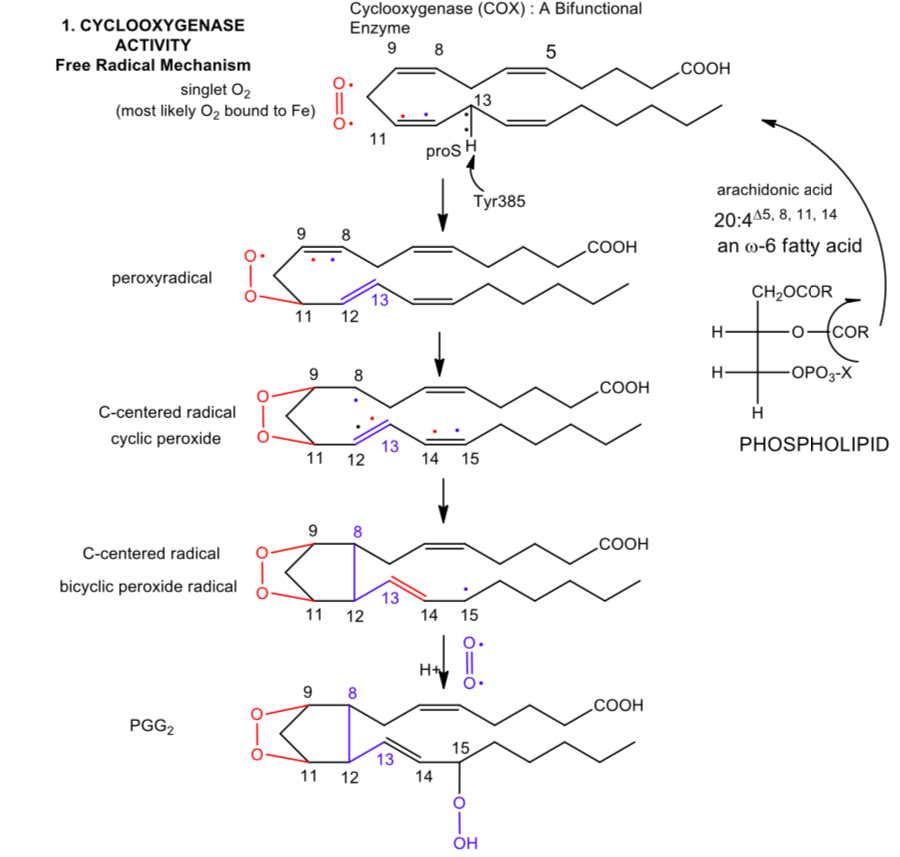
- What is the immediate oxidizing agent that starts the reaction in the scheme shown?
Cyclooxygenase (cont.)
Cyclooxygenase is a heme-containing enzyme. A tyrosine free radical initiates the reaction shown.
- What oxidizing agent might be able to abstract a hydrogen atom (an oxidation reaction) from tyrosine to form the radical?
- Given the single electron standard reduction potentials for Tyr (+0.9 V) and Fe3+in the bound heme (-0.2 to + 0.2 V for Fe3+ in various bound hemes), would the heme iron be the likely oxidizing agent?
- What possible mechanism would through the interaction of dioxygen with the heme. How might this lead to iron in a high oxidation state? Would that help initiate Tyr radical formation?
The second part of the reaction showing the peroxidase activity is shown below.
- Draw a plausible mechanism.
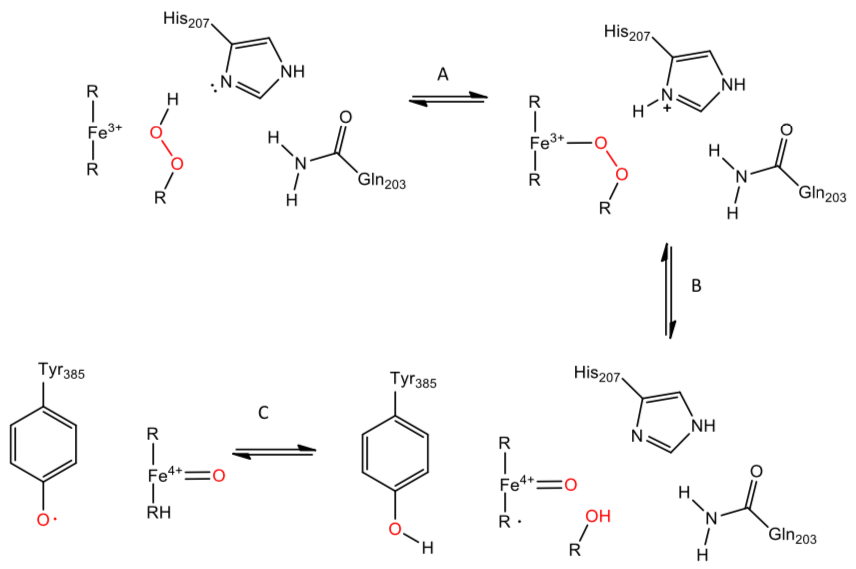
Cyclooxygenase (cont.)
A more detailed mechanism of production of the Tyr 385 free radical.
Three catalytic residues are important, Gln 203A, His 207A, and Tyr 385A, in addition to an alkyl peroxide (one of the reactant shown in the overall reaction diagram).
- Fill in reaction arrows.

Application Problems:
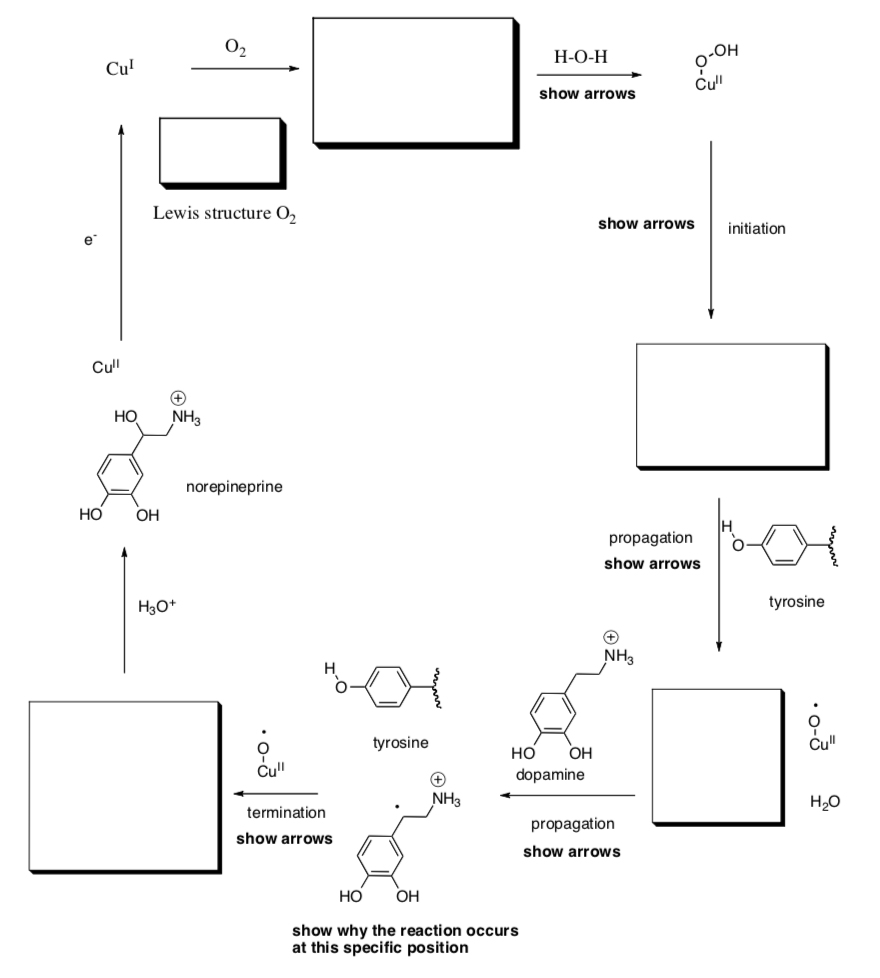
- Dopamine Monooxygenase
Dopamine b-monooxygenase is a copper-containing enzyme that helps to regulate levels of dopamine and norepinephrine. A catalytic cycle, proposed by Judith Klinman at UC Berkeley and based on kinetic isotope effect studies in her lab, involves participation of a nearby tyrosine within the enzyme.
- Fill in the boxes and follow the prompts in bold.

- Fill in the boxes and follow the prompts in bold.
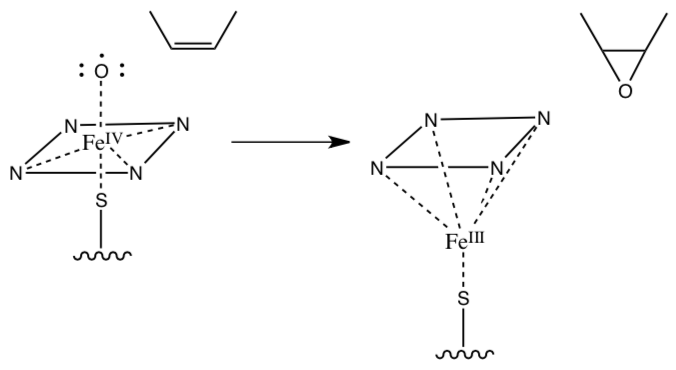
- The activated iron oxo formed of p450 has also been known to react with alkenes.
- Draw a Huckel M.O. diagram for 2-butene (structure shown in the mechanism below). Label the HOMO and LUMO.
- What effect would an additional electron do to the p-bonding on 2-butene (what would the pi bond order be)?
- Provide a mechanism for the following reaction:
- The Que lab recently synthesized the following model complex for Cytochrome P450 (Inorg. Chem. 2009, 48, 11039-11047).
- What is the oxidation state of iron in the complex? Show work in the left box (a).
- Show a typical octahedral d orbital splitting diagram in the middle box (b).
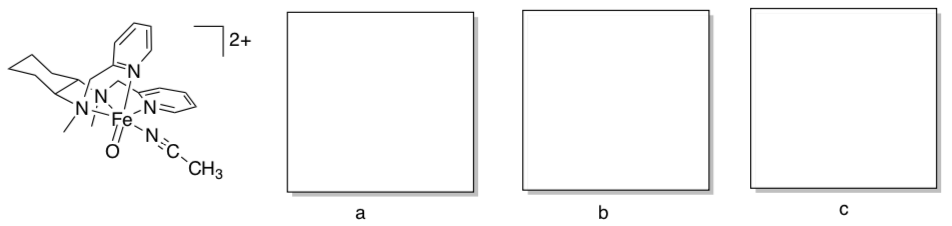
- Oxo ligands typically pi donate via two oxygen p orbitals. Modify the d orbital splitting diagram accordingly, and add appropriate electrons, in the right box (c).
- Provide an account of the electron count on iron in the complex.
DHA (see below) is often used as a substrate to probe the ability of model compounds to carry out oxidations involving C-H activation.
- Given the following reduction potential of anthracene, comment on the thermodynamics of DHA oxidation.
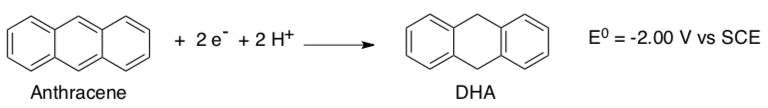
- Provide an explanation for the thermodynamics of the reaction.
- Provide a mechanism for the successful oxidation of DHA using Que’s complex.
- Fenton Reaction
Fe2+ can also react with peroxide in the Fenton reaction that can produce one of two powerful oxidants, the hydroxyl free radical in acidic solutions in a one electron step or Fe (IV) in near neutral solutions in a two electron step.
- Complete the following reaction mechanism showing production of OH radical and Fe(IV)-oxo compound, Fe(IV)=O.
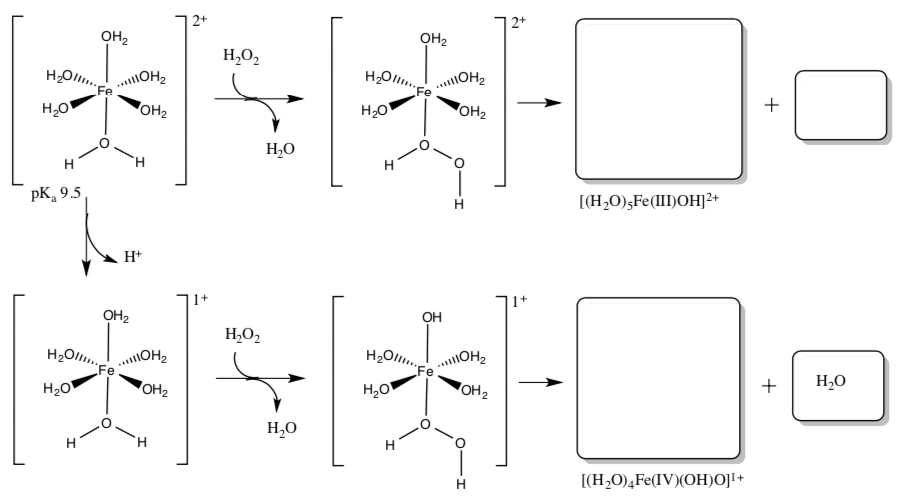
O2 reacts readily with Fe2+.
- Why does it react so quickly in comparison to dioxygen reaction with many organic substrates?
- Complete the following reaction mechanism showing production of OH radical and Fe(IV)-oxo compound, Fe(IV)=O.
- Further Adventures of Metal Oxos
Both do and d2 oxo complexes can occur as octahedral dioxo species as well (two oxo ligands on one metal). When they do, do dioxo complexes always adopt cis geometry and d2 dioxo complexes always adopt trans geometry.
- What would be the advantage of adopting cis geometry with two pi donor ligands? Explain with the help of molecular orbital cartoons and a new d orbital splitting diagram.
- Why does the d2 dioxo adopt trans geometry instead? Explain with the help of d orbital splitting diagrams for both the cis and the trans case.
- Metal oxos frequently dimerize or “polymerize” into larger structures (polyoxometallates) through oxo bridges. Show this process with Mo(O)(OR)4.
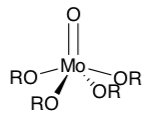
- What factors might prevent metal oxos in biology from forming extended polyoxometallate structures?
- Recently, researchers reported the isolation of an octahedral Pd(IV) oxo complex. Evaluate this claim based on your understanding of metal oxo stability.


