15: Radicals Rxns
- Page ID
- 150549
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Radical Reactions
Radicals are single-electron species. Since electrons are almost always paired in stable molecules, there is a driving force for a radical to pair up with another electron.
Radical Mechanism stages
- Initiation.
- Radicals are formed under heat or light conditions.
Radicals typically form through two different pathways: single electron transfer and bond homolysis.

- Draw the homolytic cleavage mechanism for the species below.
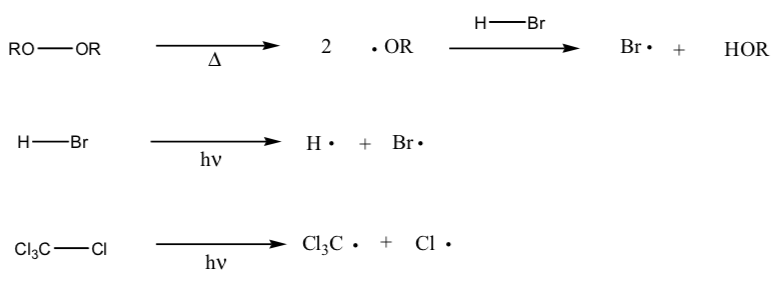
- Draw the homolytic cleavage mechanism for the species below.
- Bond Homolysis for initiation of radicals
Very weak bonds can sometimes cleave in half spontaneously at room temperature or with heat or light.
- Given the following average bond strengths (in kcal/mol), rank the top five bonds most likely to undergo homolysis.
bond Cl-Cl O-O C-N C-O Br-Br H-H C-I C-C N-N BDE 58 35 73 86 46 104 51 85 39
Benzoyl peroxide (below) is often used as a radical initiator. It is added to a reaction to provide a source of radicals.
- Show, with arrows, how this happens.
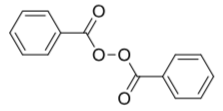
The first-formed radical from benzoyl peroxide spontaneously decarboxylates to form a phenyl radical, C6H5.
- Show how this happens using arrows.
AIBN (below) is another common radical initiator, driven by N2 formation.
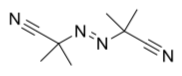
- Show, with arrows, how AIBN can cleave to form resonance-stabilized radicals and a dinitrogen molecule.
- Show a resonance structure of the radical.
- Given the following average bond strengths (in kcal/mol), rank the top five bonds most likely to undergo homolysis.
- Radicals are formed under heat or light conditions.
- Radical Propagation.
Radicals react with other species to produce new radical intermediates.
There are a number of ways for radicals to propagate. The radical may act by atom abstraction, stealing an atom (usually a hydrogen) and its electron from another molecule. It may also undergo electrophilic addition to an alkene.
- Show, with arrows, how a hydroxyl radical can abstract a hydrogen atom from a fatty acid.
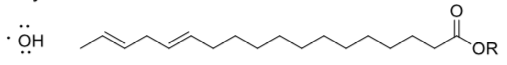
Many possible radicals may form in this event.
- Show the most likely possibility and explain why it is favoured.
- Show, with arrows, how a phenyl radical can undergo addition to a styrene.
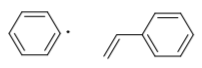
Two possible radicals may form in this event.
- Show both possibilities and explain why one is more likely.
- Show, with arrows, how the resulting radical can react with another styrene. This is how polystyrene is formed.
- Show, with arrows, how a hydroxyl radical can abstract a hydrogen atom from a fatty acid.
- Radical Termination
A termination step is any step in which radicals are destroyed, and no new radicals are produced.
Different pathways are possible. For example, two radicals may simply combine and pair up electrons together. Alternatively, one radical may perform a hydrogen atom abstraction on another.
- Show, with arrows, a growing polystyrene chain undergoing a termination step via radical recombination.
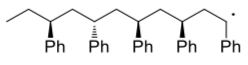
- Show, with arrows, a growing polystyrene chain undergoing a termination step via hydrogen atom abstraction.
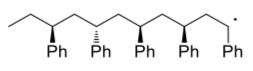
- Show, with arrows, a growing polystyrene chain undergoing a termination step via radical recombination.
Radical Bromination (put it all 3 stages together)
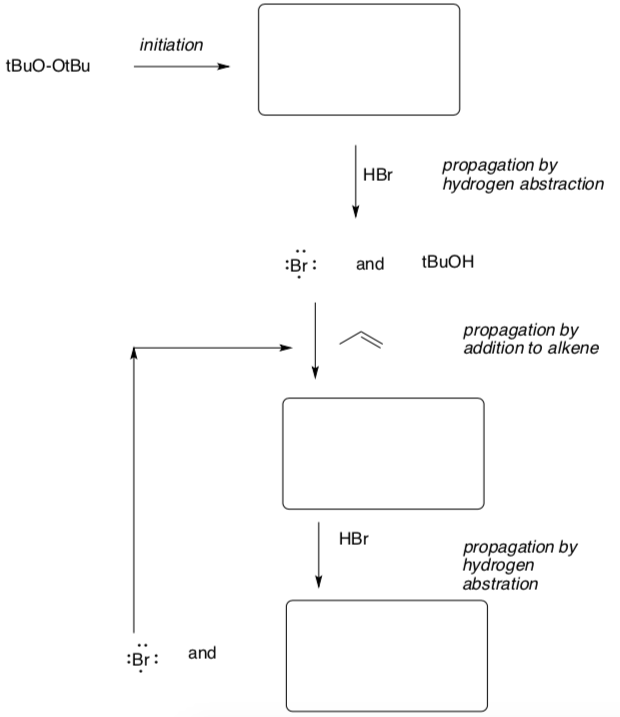
- Suggest a step that will cause termination of the chain reaction.
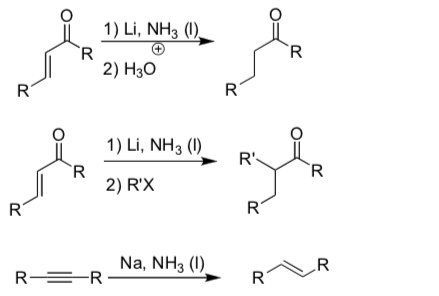
Radical reductions of alkynes
Radical reduction of alkynes is a stereospecific method for preparing trans alkenes.
- Why is sodium metal a good source of a single electron?
- Draw the arrows for this mechanism.
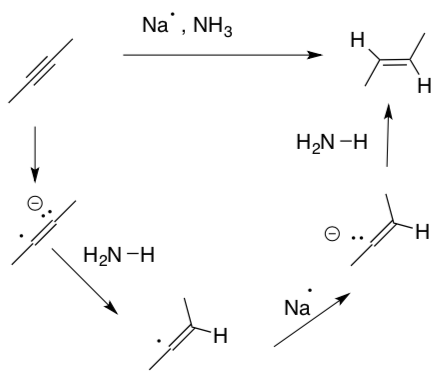
- Provide a reason for why this reaction gives only the trans product.
- Review: How do you form the cis alkene from an alkyne?
Single electron transfer in organic redox reactions
In lithium reductions of carbonyls, a single electron is transferred from the lithium atom.
- Show the intermediates generated in the following reaction.
- Add arrows to show electron movement.
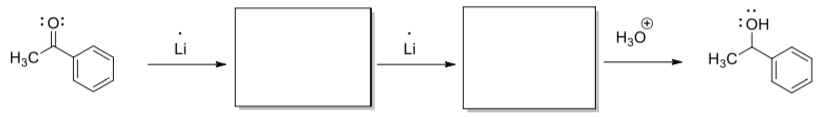
Compare the reactant on the left and product on the right.
- What is the difference in terms of number of electrons and protons in each compound?
- Why is an electron easily transferred from the lithium atom?
- Why is it so easy to transfer an electron to a carbonyl?
In black & white photography, reduction of silver salts (to silver metal) is used to produce a picture.
- Show the intermediates generated in the following reaction.
- Add arrows to show electron movement.
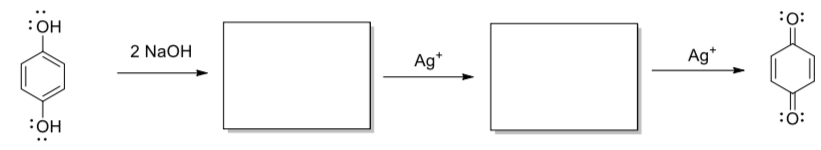
Summary of Radical Reactions
- Radical Reactions [Hint: Make Notecards]:
- Write mechanisms that explain the reactions below.
- Clearly explain regioselectivity.
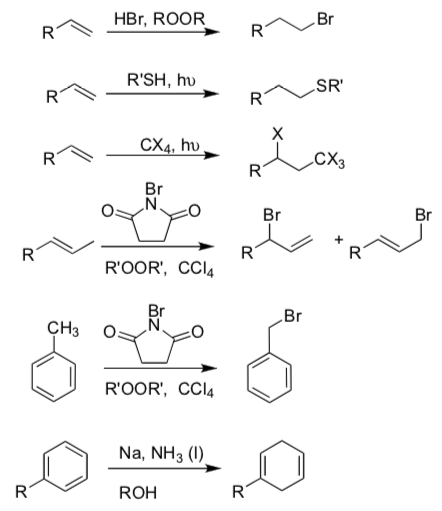
- Define the three steps of radical reactions:
- Initiation:
- Propagation:
- Termination:
Practice Radical Questions
- Provide the reagents or products for the following transformations:
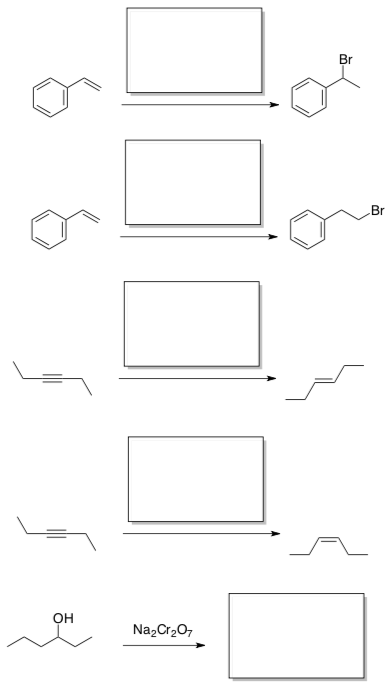
- Provide a mechanism for the following reaction:
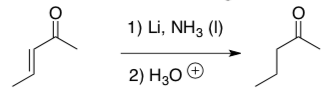
Radical Application Problem
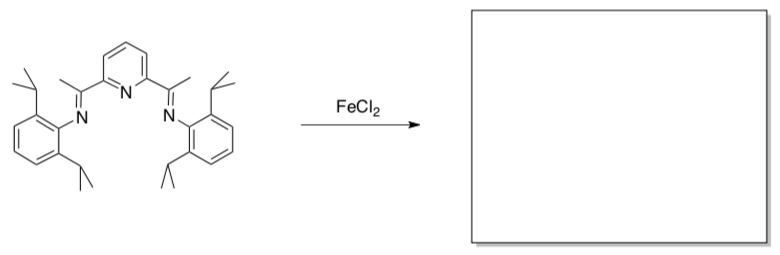
Redox-Active Ligands
Vernon Gibson (Imperial, London) reported the following synthesis of an alkene polymerization catalyst containing a pyridinediimine (PDI) ligand.
J. Am. Chem. Soc. 1999,121, 8728.
- Show the product.
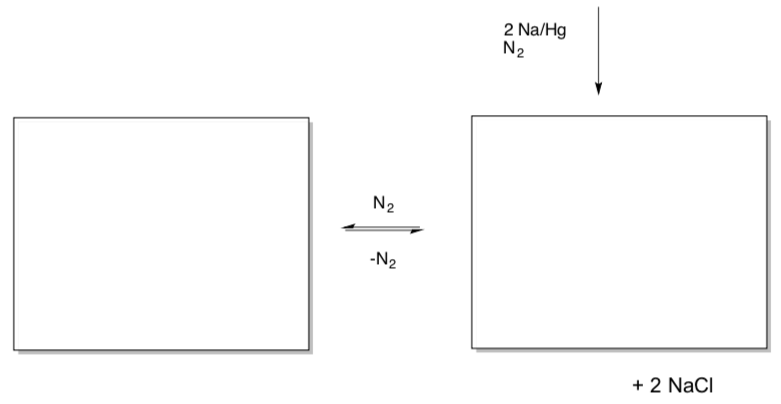
Paul Chirik (Princeton) thought this would be a good model for N2 activation. He treated the complex with sodium to obtain a dinitrogen complex (J. Am. Chem. Soc.2004,126, 13794). Under certain conditions, this complex is in equilibrium with a bis(dinitrogen complex).
- Show these two products.
- Provide a mechanism for formation of the first compound.
These compounds display unusual NMR spectra consistent with the presence of two unpaired electrons. That may be surprising given the geometry as shown by X-ray crystallography.
- Draw a d orbital splitting diagram with the appropriate number of electrons.
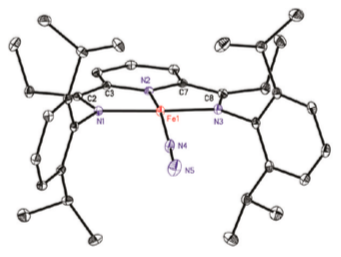
X-ray absorption spectra measure the energy of the electronic transition from a 1s energy level to an empty valence orbital (approximately). The first peak in the XAS is a good indicator of oxidation state on iron;; the peak shifts about 1 eV higher per unit increase in oxidation state. A collaboration with Karl Wieghardt (Max Planck, Mulheim) produced the following XAS data:
| compound | (PDI)FeCl2 | (PDI)FeN2 | (PDI)Fe(N2)2 | (PDI)Fe(CO)2 |
| peak1 (eV) | 7111.8 | 7111.3 | 7111.9 | 7112.4 |
- What can you conclude about the oxidation states in these compounds?
- Why does the CO complex absorb at the highest energy?
- Draw two resonance structures for (PDI)FeN2: one with Fe(II) and one with Fe(0).
There appear to be two radicals primarily based on ligand.
- Draw the ligand, and show the ligand after addition of 2 electrons. (electrophile?)
- Show that the location of the radical in the ligand is consistent with the lower of the two majority-ligand MOs.
The metal and ligand are antiferromagnetically coupled, but a low-lying triplet state is responsible for unusual NMR spectra.
Radical Polymerization Reactions
- Provide a mechanism for the following radical polymerization reaction:
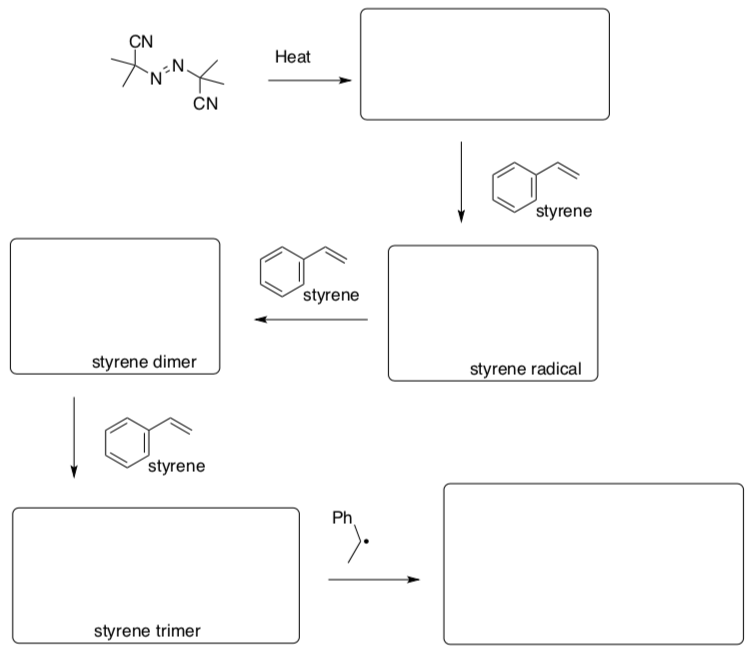
Termination Possibilities
There are a number of possible termination steps in radical polymerization. Show mechanisms for the following events.
- The radical on one growing chain combines with the radical on another (as shown in previous mechanism).
- One growing chain abstracts a hydrogen atom from the position next to the radical on another growing chain.
- One growing chain abstracts a hydrogen atom from the backbone of another growing chain (not near the radical).
- The latter case will result in a new growing chain. Show what the polymer will start to look like. (Hint: linear? Branched?)
- Termination of the polymerization can occur by exposure to air (O2). Propose a mechanism for this termination.
- Moisture is frequently a problem in these reactions. Provide a mechanism for how a stray water molecule would bring the polymerization to an end.
Living Radical Polymerization
Stable Free Radical Polymerization (SFRP) employs a radical initiator, such as benzoyl peroxide (BPO), as well as a radical trap (such as TEMPO, below).
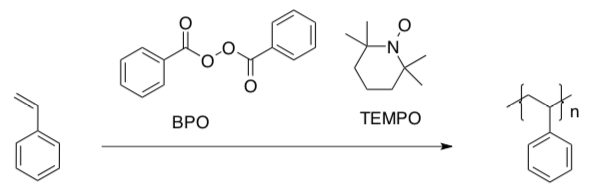
- Complete the Lewis structures for BPO and TEMPO. Note that there should be no formal charges in either molecule.
- Provide a mechanism with curved arrows for the initiation of styrene polymerization by BPO.
In a living polymerization, the polymer chain keeps growing until all monomer is consumed. The length of polymers is dependent upon the ratio of initiator to monomer feedstock.
Suppose the styrene/BPO ratio is 500.
- How many styrene molecules will one BPO react with during initiation of chain growth?
- What is the expected degree of polymerization (DP, the number of styrenes enchained in the polymer) at 100% conversion?
- What is the expected value of the average molecular weight, Mn, at 100% conversion?
- A higher than expected Mn is characteristic of early “death” of some growing chains. Explain why.
Radical Trap in Living Polymerizations
Prevention of random termination events is a crucial goal in polymer synthesis.
- Propose a rate law for a propagation step:

- Propose a rate law for a termination step:
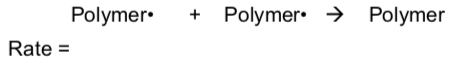
- Which type of radical reaction step is more impacted by the concentration of radicals? Circle one.
Propagation | Termination
- Random termination events are more probable at [ high / low ] radical concentration. Circle one.
- Greater control over molecular weight and morphology can be achieved by [raising / lowering ] radical concentration. Circle one.
The key approach to living radical polymerization is to generate radicals reversibly.
- Provide a mechanism with curved arrows for the reversible trapping of the growing radical chain by TEMPO (previous page).
- Explain how reversible formation of radicals helps to limit random termination steps.
Control of PDI
Random termination of polymer chain growth has an influence on the chain length as well as the distribution of chain lengths (referred to as polydispersity index, PDI, or the IUPAC term, dispersity, Đ;; the higher the dispersity, the broader the distribution). PDI impacts polymer properties.
- Explain how chain length is related to molecular weight.
- Match the following distribution curves to the descriptions:
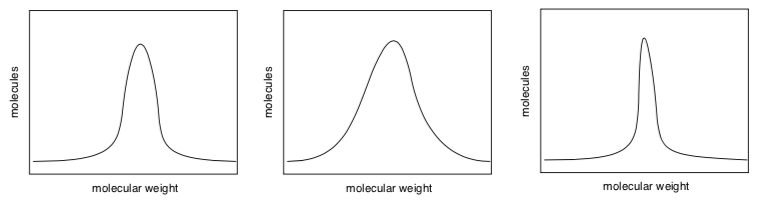
Excellent control over molecular weight / good control / poor control
Lots of random termination / some / little
PDI = 1.1 / PDI = 1.2 / PDI = 1.4
Treatment of melt styrene with varying ratios of BPO/TEMPO at 120 °C for 69 h resulted in polymers with the following characteristics.
Georges, M. K. et al;; Macromolecules 1993, 26, 2987-2988.
| TEMPO/BP O | % conversion | Mn, kD | PDI |
| 0.5 | 86 | 45.6 | 1.57 |
| 1.5 | 74 | 33.1 | 1.24 |
| 3.0 | 71 | 18.2 | 1.19 |
- Why is % conversion lower at higher ratios of TEMPO:BPO?
- Why is molecular weight lower at higher ratios of TEMPO:BPO?
- Why is PDI lower at higher ratios of TEMPO:BPO?
RAFT
Radical addition fragmentation chain transfer (RAFT) polymerization utilizes an initiator as well as a chain-transfer agent to modulate reactivity.
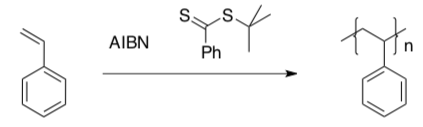
The chain transfer agent typically contains weak C-S bonds.
- Label the monomer, the initiator and the chain transfer agent in the above drawing.
At some point, reaction of the growing chain with the chain transfer agent produces a new radical via addition to a S=C bond. This step begins a process of “chain transfer”.
- Provide a mechanism, with arrows, for this process.
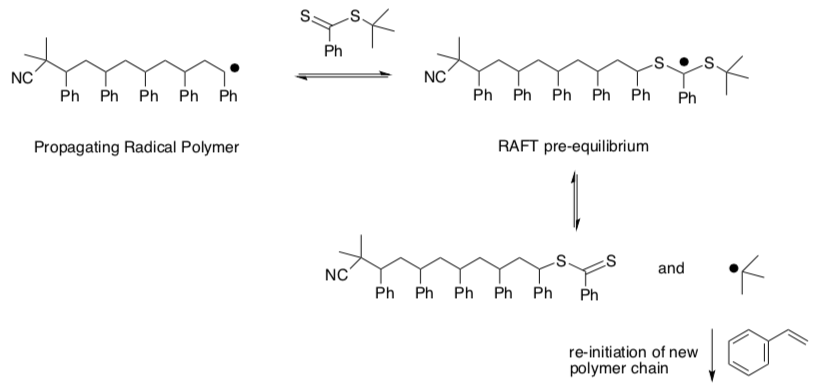
At some point, the new growing chain returns to the chain transfer agent, and the old growing chain is released again.
- Provide a mechanism with arrows.
Different RAFT agents
Fragmentation rates depend upon the group attached to the sulfur in the initial chain transfer agent. For example, in the following group, fragmentation rates vary from fastest to slowest going from left to right.
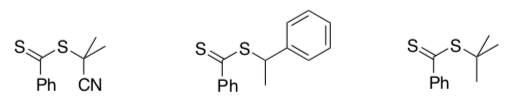
- Show the radicals resulting from fragmentation in each case.
- Explain why the fragmentation rates decrease from left to right.
In the following chain transfer agents, addition rates decrease from left to right.
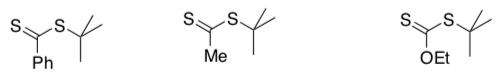
- Show the radical resulting from the addition step in each case.
- Explain why addition rates decrease from left to right.
On the other hand, in the group of radicals resulting from the addition step that you have drawn above, fragmentation rates increase from left to right.
- Explain why the fragmentation rates increase from left to right.
In order for RAFT to successfully control polymerization, the chain transfer agent must be chosen carefully. The rate of the initial fragmentation of the chain transfer agent should be competitive with the rate of polymerization (i.e. not too slow).
- Explain why.
After that, the rate of addition of growing chains to the chain transfer agent should be comparable to the rate of polymerization (i.e. not too fast if polymerization is slow, but not too slow if polymerization is fast).
- Explain why.
- Choose an optimum chain transfer agent for the following monomers (you can mix and match groups Z and R).
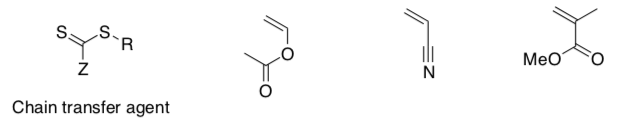
Summary of Radical Polymerizations
- List two common initiators:
- Define SFRP:
- Provide a structure of a radical trap used in SFRP.
- Define PDI and its importance for polymer chemists.
- Explain how SFRP helps control PDI.
- Define RAFT polymerization.
- Provide the structure of two different chain agents used in RAFT.
- Compare and Contrast SFRP Living Polymerization with RAFT Polymerization.
Application Problems
- Vernon Gibson (Imperial College, London) has prepared a series of iron complexes that mediate radical polymerization of alkenes. (Angew. Chem. Int. Ed. 2006, 45, 1241- 1244).
- In the compounds below
- indicate the oxidation state of iron in the space given;
- fill in the d orbital splitting diagrams, based on experimental data;
- indicate whether high or low spin;
- label the energy levels with corresponding d orbital labels.
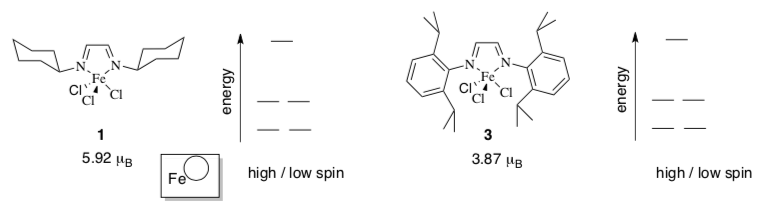
- Indicate which ligand is stronger field: 1 or 3
- Select a reason why:

- These two complexes display markedly different behaviour under radical polymerization conditions at -78oC. Complex 1 provides high molecular weight polymer of narrow dispersity, whereas complex 3 leads to early termination.
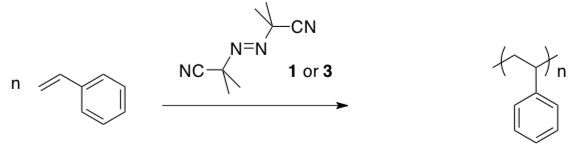
- Provide a polymerization mechanism using AIBN, NOT including termination step (also, ignore 1 or 3 for now).
- Atom Transfer Radical Polymerization moderates unwanted termination steps by “trapping” radicals reversibly, usually with a halogen radical, keeping radical concentration low.
- Show a termination step in this polymerization and indicate why it could be slowed by limiting radical concentration.
- Show how 1 or 3 could provide a halogen radical.
- Show the halogen trapping the growing polymer chain.
- Gibson has computational data that suggests that, in the case of 3, rather than being trapped by halogen, the polymer radical is trapped irreversibly by the iron species formed when a chlorine is lost from 3. The chain stops growing and eventually undergoes beta-elimination. Show these events.
- Compound 1 does not trap the polymer chain because it forms much weaker Fe-C bonds. Explain why.
- In the compounds below
- Atom transfer radical polymerization (ATRP) involves polymerization with an initiator and a redox-active metal catalyst, usually copper.
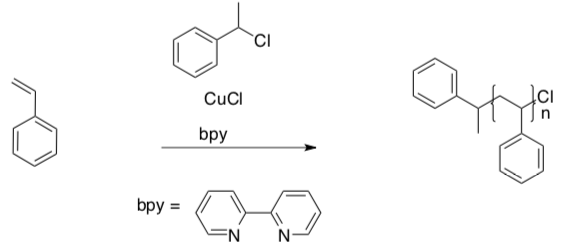
Radical initiation begins with the following reaction (called “activation”):

- What is the oxidation state of copper on each side of the equilibrium?
- Rewrite the above reaction as two balanced redox half-reactions.
After initiation, propagation of the radical ensues through polymerization, and the radical is occasionally trapped by Cl (called “deactivation”). It then waits in a dormant state until it is re-initiated by copper.
- Provide a mechanism with curved arrows for these steps.
It is important to consider the nature of the copper species that play a role in the activation and deactivation of the polymer chain. (Matyjaszewski, Coord. Chem. Rev. 2005)
A mass spectrum of a sample prepared from CuBr in the presence of 4,4’-dinonyl- 2,2’bipyridine (dNbpy) shows prominent ions at m/z = 879 and 881, in a 2:1 ratio. It is important to know that natural copper is about 67% 63Cu and about 33% 65Cu.
- Draw a structure for the cation.
- Explain the reason for the ratio of peaks in the mass spectrum.
MS can operate in reverse mode, measuring the mass of anions rather than cations. In this mode, peaks were observed in a 2:5:4:1 ratio at m/z = 221, 223, 225 and 227.
- Draw a structure for this anion.
- Explain the reason for the ratio of peaks in the mass spectrum.
As with the Cu(I) species, a number of Cu(II) species can exist in equilibrium. X-ray crystallography of one bpy complex of CuBr2 revealed a trigonal bipyramidal structure with one bromide counterion.
- Draw a structure for this coordination complex.
Based on previous experience, the electron count on copper in these complexes may seem unusual.
- Indicate the electron count in each case and say whether it seems “normal”.
Activation of the polymer chain involves a one-electron oxidation of the copper ion. This event could occur through an inner sphere or an outer sphere process.
- Draw a mechanism for outer sphere electron transfer and activation.
- Draw a mechanism for inner sphere electron transfer and activation.
The kinetics of reaction have compared to rates calculated from Marcus theory. Rate constants for initiation appear to be much faster than predicted.
- Which mechanism is more likely: inner sphere or outer sphere? Explain.
- Different Radical Traps
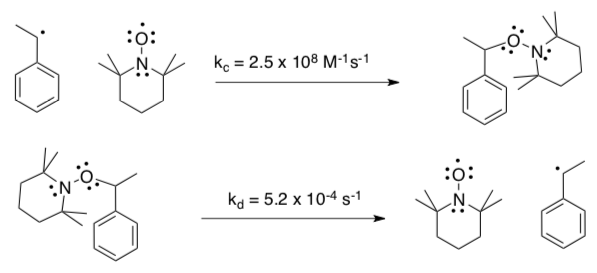
- Estimate the equilibrium constant for trapping a growing polystyrene chain with TEMPO.
Fischer also measured the following rate constants for coupling and decomposition with 1-ethylbenzene radical.
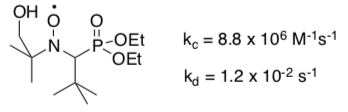
- How would this radical trap compare to TEMPO as a SFRP agent in terms of rate of polymerization of styrene?
- How would this radical trap compare to TEMPO as a SFRP agent in terms of PDI?


