19: HMBC
- Page ID
- 332819
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)HMBC*
HMBC (Heteronuclear Multiple Bond Correlation) experiment correlates chemical shifts of two types of nuclei separated from each other with two and three bonds. Older version: COLOC.
1H,13C HMBC is frequently used for assigning of quaternary and carbonyl carbons and thus connecting spin systems.
You already looked at the HMQC of ethyl (E)-but-2-enoate.
*All spectra in this unit are simulated.

| Letter | 13C Chemical Shift (ppm) | 1H Proton Shift (HMQC) |
|---|---|---|
| f | 14 | |
| a | 18 | |
| e | 62 | |
| c | 143 | |
| b | 145 | |
| d | 168 |

- Complete the table.
- Label the peaks on both axes with the appropriate letter from the table.
- Identify the two spin systems.
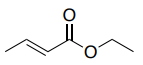
HMBC of ethyl (E)-but-2-enoate
The HMBC spectrum allows you to connect the part structures, as there are correlations to the quaternary carbon.

- Complete the table with the HMBC correlations.
| Letter | 13C Shift (ppm) | 1H Shift (HMQC) | HMBC Correlations |
|---|---|---|---|
| f | 14 | 1.2 | |
| a | 18 | 1.9 | |
| e | 62 | 4.1 | |
| c | 143 | 5.9 | |
| b | 145 | 6.95 | |
| d | 168 | -- |
- Draw all of the HMBC correlations on the structure below.
- Identify which correlation(s) allow you to connect the two spin systems.
HMBC Caveats
Three things to be aware of in HMBC spectra:
1. An HMBC is optimized for 2 and 3 bond correlations. Occasionally, 1 bond correlations are observed.
- How can you double check that you are not mistaking a 1 bond correlation for a 2- or 3-bond correlation?
2. The HMBC will occasionally show cross-peaks for 4 bonds correlations.
- When would expect to see these long-range correlations?
3. Sometimes 3 bond correlations don’t appear in the HMBC spectrum. The HMBC experiment is optimized for a particular coupling constant (~10 Hz). If the proton-carbon coupling constant is small, then the signal will be absent from the spectrum.
- When would expect that the coupling is too small? Hint: Karplus.
HMBC: Connections to Quaternary Carbons
To establish the structure of a compound with more than one spin system separated by a quaternary carbon, HMBC is invaluable.
In the structure determination of trichoderone, a cytotoxic metabolite from a marine fungus, the key piece was to determine the location of the substituents on a cyclopentenone.
J. Ind. Microbiol. Biotechnol. 2010, 37(3), 245-252.
Cyclopentenone

Substituents
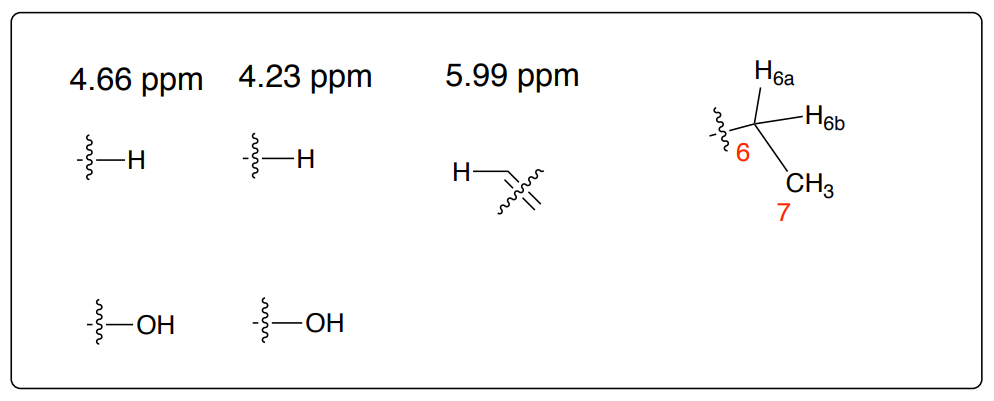
Key HH COSY Correlations:
4.23 ppm \(\leftarrow \rightarrow\) 4.66 ppm
H6a, H6b \(\leftarrow \rightarrow\) H7
H6a, H6b \(\leftarrow \rightarrow\) 5.99 ppm
5.99 ppm \(\leftarrow \rightarrow\) 4.66 ppm
| Location | 13C Shift |
|---|---|
| 1 | 202 |
| 2 | 125 |
| 3 | 180 |
| 4 | 78 |
| 5 | 81 |
| 6a | 23 |
| 6b | 23 |
| 7 | 11 |
Key HMBC Correlations:
5.99 ppm \(\rightarrow\) C1, C2, C3, C4, C5
H6a, H6b \(\rightarrow\) C2, C3, C4
H7 \(\rightarrow\) C3
- Propose a structure for trichoderone.
- Draw the HMBC correlations as double-headed arrows on your structure.
HMBC to Distinguish Isomers
The ability to connect spin systems is quite powerful especially when looking at isomers.
When looking at the 1H NMR and 13C NMR of this compound, one can propose three spin systems.

You could propose two possible structures containing these spin systems and an ester.
 ethyl 3-methoxypropanoate
ethyl 3-methoxypropanoate
or
 methyl 3-ethoxypropanoate
methyl 3-ethoxypropanoate
The HMQC and HMBC spectra are on the following pages.
- Fill in the following table.
- Draw the HMBC correlations as double-headed arrows on the structures.
- Determine which of the two isomers best fits the data.
| Letter | 13C Shift (ppm) | 1H Shift (HMQC) | HMBC Correlations |
|---|---|---|---|




