14: COSY
- Page ID
- 332814
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)COSY Spectroscopy*
Imagine if you could do the homonuclear spin decoupled experiment for all hydrogens in the molecule in one spectrum.
COSY is a 2D NMR experiment (homonuclear COrrelation SpectroscopY) that runs decoupling pulses along the entire frequency range and records all coupling.
This technique is used to identify spins which are coupled to each other (usually up to four bonds).
A cross-peak always shows up along the diagonal, where a peak on one axis corresponds with the identical peak on the other (“self-correlations”, in black). These are simply the 1H spectra.
- Draw a line through the diagonal.
Correlations that represent coupling show up both above and below the diagonal. In this spectrum, there are two correlations in red and blue.
- Use a ruler to connect these correlations to the original peaks (black on the diagonal) and the chemical shifts.
These correlations are usually represented with double headed arrows on the structure and placed in a new column on our data tables.

* All spectra are either from SDBS (Japan National Institute of Advanced Industrial Science and Technology) or simulated.
2-Butanone: An Introduction to COSY
The 1H NMR Spectrum of 2-butanone (C4H8O) is shown below.


COSY Spectrum for 2-butanone
Diagonal peaks correspond to the peaks in a 1D-NMR experiment.
- Draw a line through the diagonal.
The cross peaks (correlations)indicate couplings between pairs of nuclei.
- Draw the lines between the correlations and the shift.
- Draw the correlations on the structure above with doubleheaded arrows.

When there is no coupling, no correlation is expected to appear.
- Identify the peak that has no correlations. Which set of hydrogens is this?
Draw Your Own COSY
The 1H spectrum of iodobutane is shown below:

- Predict the COSY spectrum for this structure.

Distinguish Isomers using COSY
The 1H NMR Spectrum of Compound A (C10H10O2) is below.

- The singlet at 3.8 ppm is ____________.
- The position of the aldehydic H at _______ ppm can easily allow for the assignment of the peaks in the H atoms on the double bond in the straight chain.
- There also appears to be an alkene at (_________ ppm) and an aromatic ring at (_________ ppm )
But the connectivity is a little bit trickier as you could imagine two possible structures:
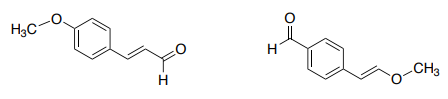
The COSY on the next page will allow us to see correlations.
Distinguish Isomers using COSY (cont.)
Sometimes, COSY or 2D correlation spectroscopy shows more correlations than are visible in 1D spectrum. Thus, you will occasionally see correlations to an apparent singlet.
COSY Spectrum of C10H10O2.

- Label each peak in the COSY.
- Show the peaks that are correlated to each other.
- Which is the correct structure?

COSY to Distinguish Overlapping Peaks
It is particularly useful when there are overlapping peaks in the 1D 1H NMR.
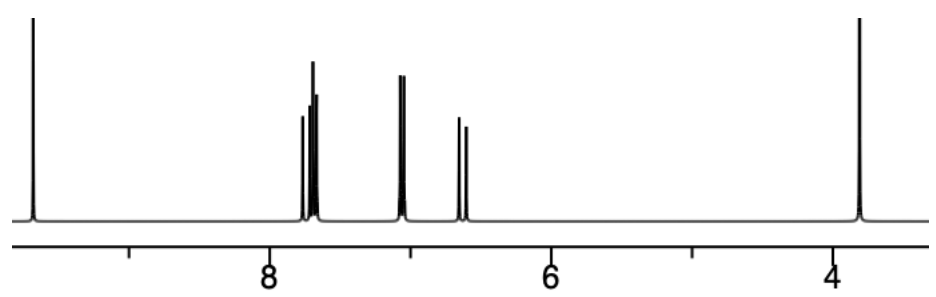
This is the 1H NMR of an isomer of octanone.
Note: the peaks at 2.43 and 1.56 ppm are both peaks from two different CH2 groups. This makes it difficult to interpret the coupling.

- Complete the following table:
| Shift (ppm) | Integration | Multiplicity |
|---|---|---|
| 2.43 | ||
| 1.56 | ||
| 1.35 | ||
| 1.09 | ||
| 0.91 |
COSY to Distinguish Overlapping Peaks (cont.)
COSY helps to clearly identify the peaks that are coupled even when the peaks are close together.
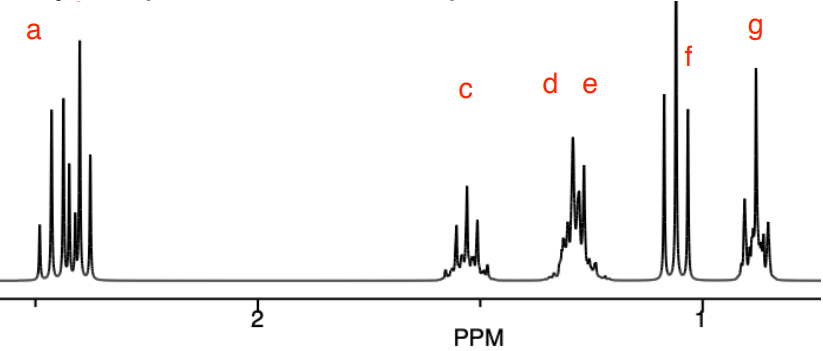

Note: This is a simulated spectrum; in a real spectrum, the cross peaks might not be as easily distinguished.
- Complete the following table:
- Choose the correct isomer based on the coupling.
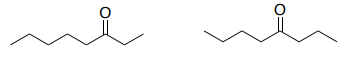
- Draw the correlations on the correct structure using double-headed arrows.
| Shift (ppm) | Label | COSY Correlations |
|---|---|---|
| 2.43 |
a b |
|
| 1.56 | c | |
| 1.35 |
d e |
|
| 1.09 | f | |
| 0.91 | g |
Analyzing the Aromatic Substitution Pattern with COSY
The COSY spectrum of dinitrobenzene is shown below.

- Determine the substitution pattern (ortho, meta or para). Circle one below.

- Show your correlations using double-headed arrows.
Structural Problems with COSY
Compound 1
The IR, 13C & 1H NMR spectrum of 1 is shown below as well the COSY.
- MF: ________
- SU:_________
- Complete the tables provided.
- Determine the structure of C7H7N.
- Show COSY correlations with doubleheaded arrows.
| Frequency | Functional Group |
|---|---|
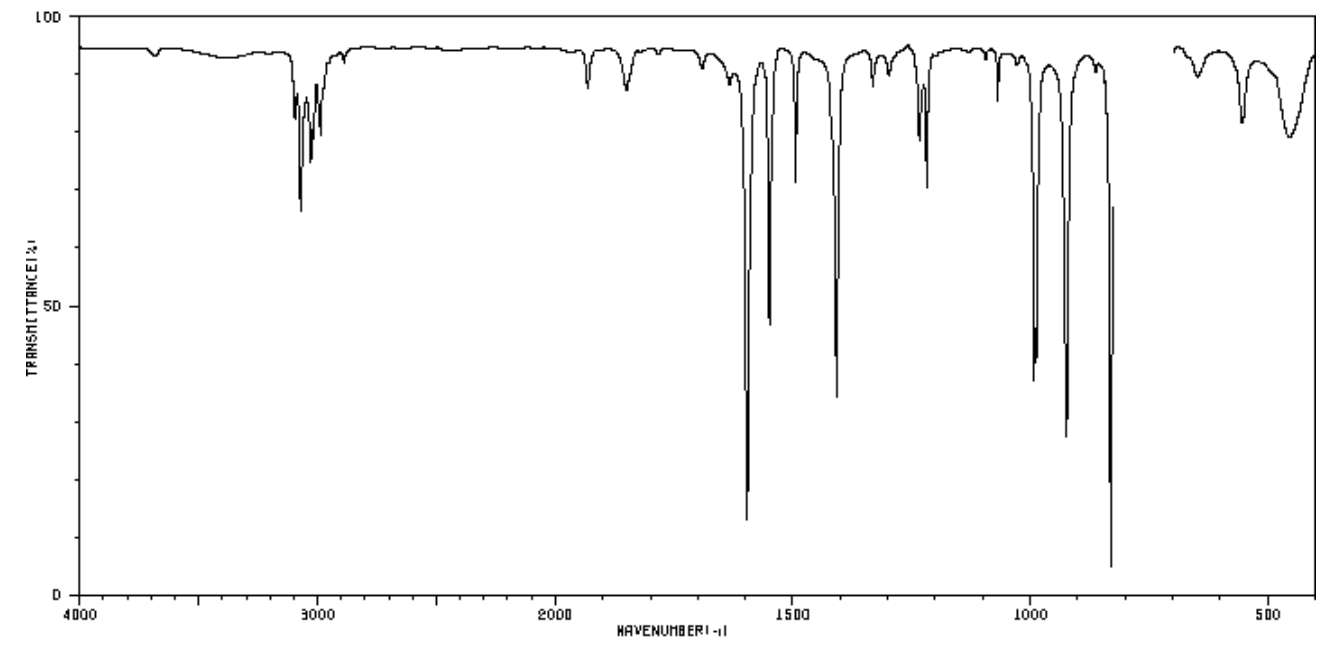

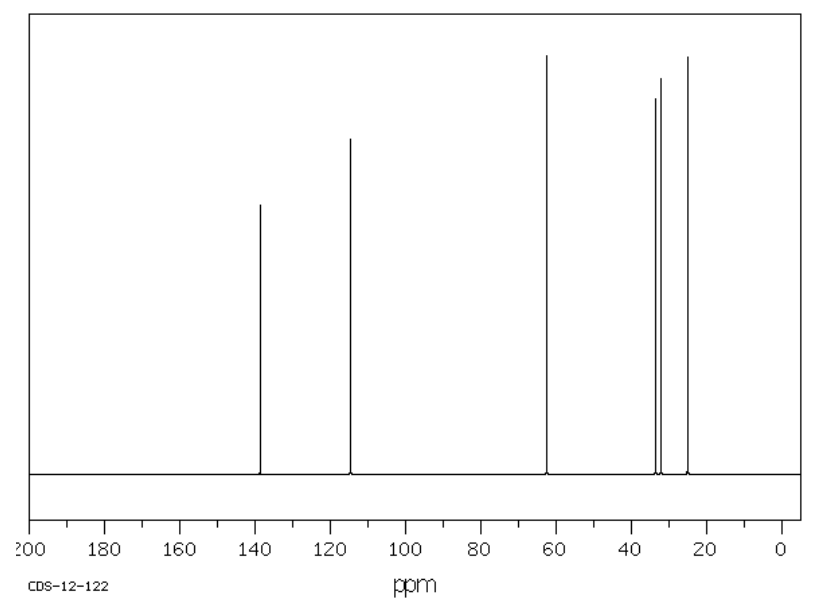
| Carbon Shift | Assignment |
|---|---|
Compound 1 (continued)
1D 1H NMR
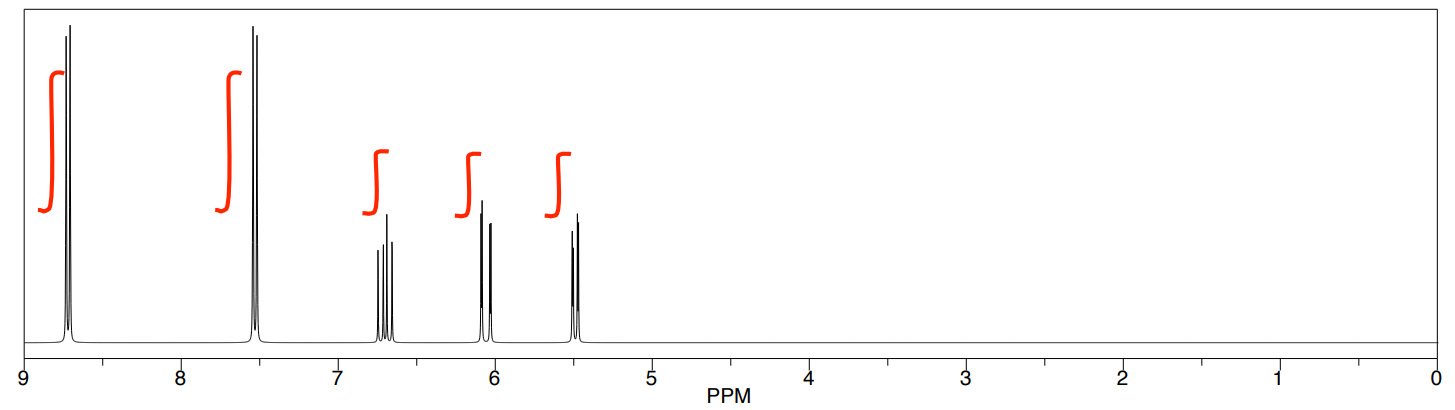
1H with COSY Correlations (COSY on next page)
| Shift (ppm) | Letter | #H | Mult. | 3JHH | Part Structure |
|---|---|---|---|---|---|
- Proposed Structure
- Include Correlations with Arrows.
Compound 1 (continued)
COSY for Compound 1

Compound 2
The IR, 13C & 1H NMR spectrum of 2 is shown below as well the COSY.
- MF: ________
- SU:_________
- Complete the tables provided.
- Determine the structure of C6H12O.
- Show COSY correlations with doubleheaded arrows.
| Frequency | Functional Group |
|---|---|

| Carbon Shift | Assignment |
|---|---|
Compound 2 (continued)
1D 1H NMR

1H with COSY Correlations (COSY on next page:
| Shift (ppm) | Letter | #H | Mult. | 3JHH | Part Structure |
|---|---|---|---|---|---|
- Proposed Structure
- Include Correlations with Arrows.
Compound 2 (continued)
COSY for Compound 2



