17: VSEPR Theory and Shapes of Molecules (Experiment)
- Page ID
- 95997
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To build a variety of molecules and ions using molecular model kits.
- To draw Lewis structures (both projection and perspective drawings) for each of these molecules and ions.
- To determine the hybridization of the central atoms, the number and types of bonds, the geometries, and the polarities of the molecules and ions.
Lewis Structures
A Lewis Structure is a representation of covalent molecules (or polyatomic ions) where all the valence electrons are shown distributed about the bonded atoms as either shared electron pairs (bond pairs) or unshared electron pairs (lone pairs). A shared pair of electrons is represented as a short line (a single bond). Sometimes atoms can share two pairs of electrons, represented by two short lines (a double bond). Atoms can even share three pairs of electrons, represented by three short lines (a triple bond). Pairs of dots are used to represent lone pair electrons. Examples are shown for the molecules \(ce{SF2}\) and \(\ce{CH2O}\) below.
Please review (in your text or notes) the rules for drawing Lewis structures before performing this exercise. This includes rules for structures which obey the octet rule, as well as those which involve expanded or reduced octets.
Resonance Structures
Resonance refers to bonding in molecules or ions that cannot be correctly represented by a single Lewis structure. These structures are often equivalent, meaning that they contain the same number of bonds at different locations. The molecule \(\ce{SO2}\) (shown above) has two such resonance forms. Resonance structures can also be non-equivalent, in which case they will have different numbers and/or locations of bonds. Note that any valid resonance structure of a molecule can be used to determine its shape and polarity.
VSEPR Theory
The VSEPR (Valence Shell Electron Pair Repulsion) model is used to predict the geometry of molecules based on the number of effective electron pairs around a central atom. The main postulate for the VSEPR theory is that the geometrical structure around a given atom is principally determined by minimizing the repulsion between effective electron pairs. Both the molecular geometry and the polarity of individual bonds then determine whether the molecule is polar or not.
Before determining the shape of a molecule, the Lewis structure must be properly drawn. The shape of a molecule is then determined by the number of areas of electron density (or, number of effective electron pairs) around a central atom. Areas of electrons density include:
- Lone pairs of electrons: these electrons tend to take more space than the bonded pairs in space leading into somewhat distorted structures.
- Bonds (single, double and triple bonds count as one (1) area of electron density or one effective electron pair).
Before performing this exercise, please review (in your text or notes) the various geometries and bond angles that can be produced by different numbers of effective electron pairs around the central atom.
Molecular Polarity
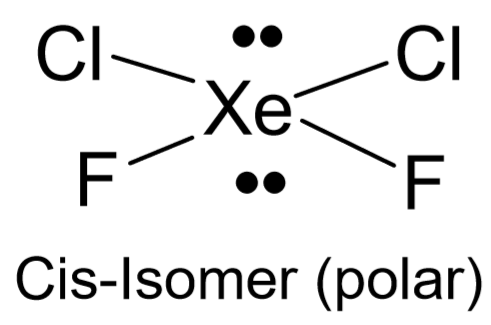
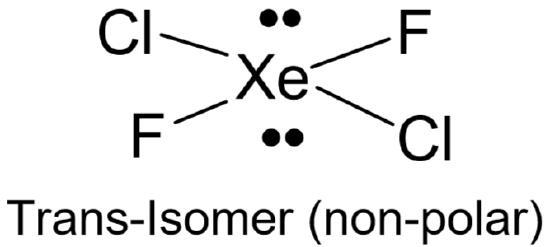
A polar bond is one in which the electron cloud is closer to the nucleus of one atom (the more electronegative one) than the other (the less electronegative one). Knowledge of both the bond polarities and the shape are required in the determination of the molecule’s overall polarity (dipole moment). A polar molecule is one that shows an imbalance in its electron distribution. When placed in an electric field, these molecules tend to align themselves with the electric field. Some molecules have polar bonds but no dipole moment. This happens when the bonds in a molecule are arranged in a way in which polarities cancel each other out. One way this occurs is when molecules have all identical bonds and there is no lone pair on the central atom (for example, \(\ce{CO2}\)). Molecules that do not fit both these criteria may be polar or not depending on how atoms are bonded and the electron pairs arranged around the central atom (for example, \(\ce{XeCl2F2}\) shown below).
Procedure
Materials and Equipment
Molecular model kits from the stockroom
Instructions
Complete the following for each of the molecules and ions on your Report form:
- Draw Lewis structures, including all resonance structures if applicable (1).
- Build models and then draw perspective structures (2) that accurately represent bond angles and molecular shapes.
The molecular model kits contain different colored balls and different size stick connectors. Three-dimensional models will be constructed from these balls and sticks.
The stick connectors represent bonds. Use the short rigid sticks for single bonds. The long flexible sticks must be used to create double (2 sticks) and triple (3 sticks) bonds.
The different colored balls represent different atoms. There is a key on the inside of the lid of the model kit which indicates which colors correspond to which atoms. There are, however, some exceptions to this. For example, the kit indicates that the green balls with just one hole are to be used for the halogens. But if a halogen (such as \(\ce{Cl}\)) appears in a molecule as a central atom with an expanded octet, you would need to use a ball that has 5 or 6 holes to build the model (brown or silver ball). Another example is oxygen. The kit indicates that the red balls with two holes should be be used for oxygen. However, if an oxygen atom in a compound requires more than two bonds, the red balls cannot be used. In this case you would substitute a blue ball for oxygen.
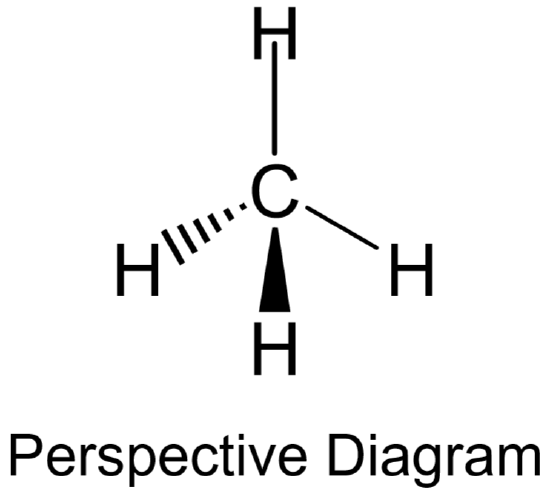
The 3-D models will serve as a visual guide to help you with your perspective structures. Use the following guidelines to draw them correctly:
- For bonds lying in the plane of the paper, use a regular solid line.
- For bonds that project down into the paper away from you, use a hatched wedge-shaped line (
 ).
). - For bonds that project up out of the paper towards you, use a solid wedge-shaped line (
 ).
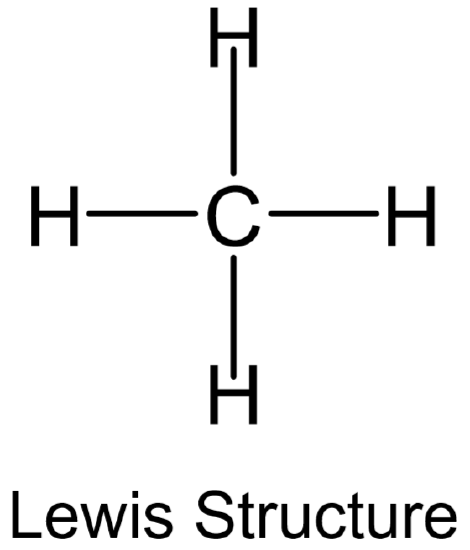
). - An example showing both the Lewis structure and perspective representation of\(\ce{CH4}\) is provided below.
- Determine the number of atoms bonded to the central atom (or, number of \(\sigma \)-bonds) (3).
- Determine the number of lone electron pairs on the central atom (4).
- Predict the electronic geometry using all areas of electron density (or, effective electron pairs) and the ideal bond angles associated with this geometry (5).
- Predict the actual geometry of the molecule or ion (6).
- Determine the hybridization of the central atom (7).
- Determine the polarity of the molecule (8). Use an arrow to show the direction of electron density for polar molecules on the perspective drawing.
Please be sure to return all balls and stick connectors to the model kit when finished.
Pre-laboratory Assignment: VSEPR Theory and Shapes of Molecules
Complete the following table
|
Chemical Species |
\(\ce{KrF2}\) |
\(\ce{PH4^+}\) |
\(\ce{TeCl6}\) |
\(\ce{ClBr3}\) |
\(\ce{H2S}\) |
|---|---|---|---|---|---|
|
Lewis Structure |
|||||
|
Perspective Drawing |
|||||
|
Number of atoms bonded to central atom |
|||||
|
Number of lone pairs on central atom |
|||||
|
Electronic geometry |
|||||
|
Molecular Geometry |
|||||
|
Polarity |
Lab Report: VSEPR Theory and Shapes of Molecules
\(\ce{HCN}\):
|
1. Lewis Structure |
2. Perspective drawing | ||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{CH3OH}\):
|
1. Lewis Structure |
2. Perspective drawing |
||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{SeF6}\):
|
1. Lewis Structure |
2. Perspective drawing | ||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{NO2^-}\):
|
1. Lewis Structure |
2. Perspective drawing |
||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{AsF5}\):
|
1. Lewis Structure |
2. Perspective drawing | ||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{XeF2}\):
|
1. Lewis Structure |
2. Perspective drawing |
||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{TeF5^-}\):
|
1. Lewis Structure |
2. Perspective drawing | ||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{H2CO}\)
|
1. Lewis Structure |
2. Perspective drawing |
||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{SF4}\)
|
1. Lewis Structure |
2. Perspective drawing | ||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{XeF4}\)
|
1. Lewis Structure |
2. Perspective drawing |
||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{PO4^{3-}}\)
|
1. Lewis Structure |
2. Perspective drawing | ||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{BrF3}\)
|
1. Lewis Structure |
2. Perspective drawing |
||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{NH3}\)
|
1. Lewis Structure |
2. Perspective drawing |
||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
\(\ce{CH3NH2}\)
|
1. Lewis Structure |
2. Perspective drawing |
||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
Molecule:
|
1. Lewis Structure |
2. Perspective drawing | ||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
Molecule:
|
1. Lewis Structure |
2. Perspective drawing |
||
|
3. Number of atoms bonded to central atom |
4. Number of non-bonding electron pairs on the central atom |
5. Electronic geometry: |
|
|
6. Molecular geometry with ideal bond angles |
7. Hybridization of central atom |
8. Polarity: |
|
Questions
- For each one of the molecules with lone pairs of electrons on the central atom that is non polar, give an explanation why they have no dipole moment.
- Find all molecules (or ions) with resonance structures and draw them in the box below:
| 1 | |||
|---|---|---|---|
| 2 | |||
| 3 |
Summary of Types of Shapes
|
Areas of electron density |
Molecular geometry |
Example of species |
Polarity of the example | ||
|---|---|---|---|---|---|
|
Number atoms bonded to cenral atom (\(\sigma\)-bonds) |
Number of lone pairs |
Electronic geometry | |||
|
2 |
0 |
||||
|
3 |
0 |
||||
|
2 |
1 |
||||
|
4 |
0 |
||||
|
3 |
1 |
||||
|
2 |
2 |
||||
|
5 |
0 |
||||
|
4 |
1 |
||||
|
3 |
2 |
||||
|
2 |
3 |
||||
|
6 |
0 |
||||
|
5 |
1 |
||||
|
4 |
2 |
||||